Omron Healthcare HEM-7361T-EBK Manuale utente
- Categoria
- Unità di pressione sanguigna
- Tipo
- Manuale utente

Instruction Manual
1
EN
FR
DE
IT
ES
NL
RU
TR
AR
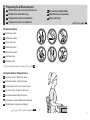
Symbols
Automatic Upper Arm Blood Pressure Monitor
M7 Intelli IT (HEM-7361T-EBK)
X7 Smart (HEM-7361T-ESL)
Read Instruction manual and before use.
FR
Lire le mode d’emploi et avant l’utilisation.
DE
Lesen Sie vor der Verwendung Gebrauchsanweisung und .
IT
Leggere il manuale di istruzioni e prima dell’uso.
ES
Lea el manual de instrucciones y antes del uso.
NL
Lees gebruiksaanwijzing en voor gebruik.
RU
.
TR
Kullanmadan önce, kullanım kılavuzu ve 'yi okuyun.
AR
Symboles/ Symbole/ Simboli/ Símbolos/
Symbolen/ / Semboller/

EN1
1. Introduction
Thank you for purchasing the OMRON Automatic Upper Arm Blood Pressure
Monitor. This blood pressure monitor uses the oscillometric method of blood
pressure measurement. This means this monitor detects your blood movement
through your brachial artery and converts the movements into a digital reading.
1.1 Safety Instructions
This instruction manual provides you with important information about the
OMRON Automatic Upper Arm Blood Pressure Monitor. To ensure the safe
and proper use of this monitor, READ and UNDERSTAND all of the safety and
operating instructions. If you do not understand these instructions or have
any questions, contact your OMRON retail outlet or distributor before
attempting to use this monitor. For specific information about your own
blood pressure, consult with your physician.
1.2 Intended Use
The device is a digital monitor intended for use in measuring blood pressure
and pulse rate in adult patient population. The device detects the appearance
of irregular heartbeats during measurement and gives a warning signal with
readings. It is mainly designed for general household use.
The device can detect an irregular pulse suggestive of Atrial Fibrillation
(Afib).
Please note that the device is not intended to diagnose Afib. A diagnosis of Afib
can only be confirmed by Electrocardiogram (ECG). If the Afib symbol appears,
consult your physician.
1.3 Receiving and Inspection
Remove this monitor and other components from the packaging and inspect
for damage. If this monitor or any other components is damaged, DO NOT USE
and consult with your OMRON retail outlet or distributor.
2. Important Safety Information
Read the Important Safety Information in this instruction manual before using
this monitor. Follow this instruction manual thoroughly for your safety.
Keep for future reference. For specific information about your own blood
pressure, CONSULT WITH YOUR PHYSICIAN.
2.1 Warning
Indicates a potentially hazardous situation
which, if not avoided, could result in death or
serious injury.
• DO NOT use this monitor on infants, toddlers, children or persons who
cannot express themselves.
• DO NOT adjust medication based on readings from this blood pressure
monitor. Take medication as prescribed by your physician. ONLY a physician
is qualified to diagnose and treat high blood pressure and Afib.
• DO NOT use this monitor on an injured arm or an arm under medical treatment.
• DO NOT apply the arm cuff on your arm while on an intravenous drip or
blood transfusion.
• DO NOT use this monitor in areas containing high frequency (HF) surgical
equipment, magnetic resonance imaging (MRI) equipment, computerized
tomography (CT) scanners. This may result in incorrect operation of the
monitor and/or cause an inaccurate reading.
• DO NOT use this monitor in oxygen rich environments or near flammable gas.
• Consult with your physician before using this monitor if you have common
arrhythmias such as atrial or ventricular premature beats or atrial fibrillation;
arterial sclerosis; poor perfusion; diabetes; pregnancy; pre-eclampsia or renal
disease. NOTE that any of these conditions in addition to patient motion,
trembling, or shivering may affect the measurement reading.
• NEVER diagnose or treat yourself based on your readings. ALWAYS consult
with your physician.
• To help avoid strangulation, keep the air tube and AC adapter cable away
from infants, toddlers and children.
• This product contains small parts that may cause a choking hazard if
swallowed by infants, toddlers and children.
Data Transmission
• This product emits radio frequencies (RF) in the 2.4 GHz band. DO NOT use
this product in locations where RF is restricted, such as on an aircraft or in
hospitals. Turn off the Bluetooth® feature in this monitor, remove batteries
and/or unplug the AC adapter when in RF restricted areas.
AC Adapter (optional accessory) Handling and Usage
• DO NOT use the AC adapter if this monitor or the AC adapter cable is
damaged. If this monitor or the cable is damaged, turn off the power and
unplug the AC adapter immediately.
• Plug the AC adapter into the appropriate voltage outlet. DO NOT use in a
multi-outlet plug.
• NEVER plug in or unplug the AC adapter from the electric outlet with wet hands.
• DO NOT disassemble or attempt to repair the AC adapter.
Battery Handling and Usage
• Keep batteries out of the reach of infants, toddlers and children.
2.2 Caution
Indicates a potentially hazardous situation which, if
not avoided,may result in minor or moderate injury
to the user or patient, or cause damage to the
equipment or other property.
• Stop using this monitor and consult with your physician if you experience
skin irritation or discomfort.
• Consult with your physician before using this monitor on an arm where
intravascular access or therapy, or an arteriovenous (A-V) shunt, is present
because of temporary interference to blood flow and could result in injury.
• Consult with your physician before using this monitor if you have had a mastectomy.
EN

EN2
EN
• Consult with your physician before using this monitor if you have severe
blood flow problems or blood disorders as cuff inflation can cause bruising.
• DO NOT take measurements more often than necessary because bruising,
due to blood flow interference, may occur.
• ONLY inflate the arm cuff when it is applied on your upper arm.
• Remove the arm cuff if it does not start deflating during a measurement.
• DO NOT use this monitor for any purpose other than measuring blood pressure
and/or detecting the possibility of Afib.
• During measurement, make sure that no mobile device or any other
electrical device that emit electromagnetic fields is within 30 cm of this
monitor. This may result in incorrect operation of the monitor and/or cause
an inaccurate reading.
• DO NOT disassemble or attempt to repair this monitor or other components.
This may cause an inaccurate reading.
• DO NOT use in a location where there is moisture or a risk of water splashing
this monitor. This may damage this monitor.
• DO NOT use this monitor in a moving vehicle such as in a car or on an aircraft.
• DO NOT drop or subject this monitor to strong shocks or vibrations.
• DO NOT use this monitor in places with high or low humidity or high or low
temperatures. Refer to section 6.
• During measurement, observe the arm to ensure that the monitor is not
causing prolonged impairment to blood circulation.
• DO NOT use this monitor in high-use environments such as medical clinics or
physician offices.
• DO NOT use this monitor with other medical electrical (ME) equipment
simultaneously. This may result in incorrect operation of the monitor and/or
cause an inaccurate reading.
• Avoid bathing, drinking alcohol or caffeine, smoking, exercising and eating
for at least 30 minutes before taking a measurement.
• Rest for at least 5 minutes before taking a measurement.
• Remove tight-fitting, thick clothing and any accessories from your arm while
taking a measurement.
• Remain still and DO NOT talk while taking a measurement.
• ONLY use the arm cuff on persons whose arm circumference is within the
specified range of the cuff.
• Ensure that this monitor has acclimated to room temperature before taking
a measurement. Taking a measurement after an extreme temperature
change could lead to an inaccurate reading. OMRON recommends waiting
for approximately 2 hours for the monitor to warm up or cool down when
the monitor is used in an environment within the temperature specified
as operating conditions after it is stored either at the maximum or at the
minimum storage temperature. For additional information on operating and
storage / transport temperature, refer to section 6.
• DO NOT use this monitor after the durable period has ended. Refer to section 6.
• DO NOT crease the arm cuff or the air tube excessively.
• DO NOT fold or kink the air tube while taking a measurement. This may cause
an injury by interrupting blood flow.
• To unplug the air plug, pull on the plastic air plug at the base of the tube, not
the tube itself.
• ONLY use the AC adapter, arm cuff, batteries and accessories specified for
this monitor. Use of unsupported AC adapters, arm cuffs and batteries may
damage and/or may be hazardous to this monitor.
• ONLY use the approved arm cuff for this monitor. Use of other arm cuffs may
result in incorrect readings.
• Inflating to a higher pressure than necessary may result in bruising of the arm
where the cuff is applied. NOTE: refer to “If your systolic pressure is more than
210mmHg” in section 13 of instruction manual
for additional information.
• Read and follow the “Correct Disposal of This Product” in section 7 when
disposing of the device and any used accessories or optional parts.
Data Transmission
• DO NOT replace batteries or unplug the AC adapter while your readings are
being transferred to your smart device. This may result in incorrect operation
of this monitor and failure to transfer your blood pressure data.
AC Adapter (optional accessory) Handling and Usage
• Fully insert the AC adapter into the outlet.
• When unplugging the AC adapter from the outlet, be sure to safely pull from
the AC adapter. DO NOT pull from the AC adapter cable.
• When handling the AC adapter cable:
Do not damage it. / Do not break it. / Do not tamper with it. / DO NOT pinch
it. / Do not forcibly bend or pull it. / Do not twist it. / DO NOT use it if it is
gathered in a bundle. / DO NOT place it under heavy objects.
• Wipe any dust off of the AC adapter.
• Unplug the AC adapter when not in use.
• Unplug the AC adapter before cleaning this monitor.
Battery Handling and Usage
• DO NOT insert batteries with their polarities incorrectly aligned.
• ONLY use 4 “AA” alkaline or manganese batteries with this monitor. DO NOT
use other types of batteries. DO NOT use new and used batteries together.
DO NOT use different brands of batteries together.
• Remove batteries if this monitor will not be used for a long period of time.
• If battery fluid should get in your eyes, immediately rinse with plenty of clean
water. Consult with your physician immediately.

EN3
• If battery fluid should get on your skin, wash your skin immediately with
plenty of clean, lukewarm water. If irritation, injury or pain persists, consult
with your physician.
• DO NOT use batteries after their expiration date.
• Periodically check batteries to ensure they are in good working condition.
2.3 General Precautions
• When you take a measurement on the right arm, the air tube should be at
the side of your elbow. Be careful not to rest your arm on the air tube.
• Blood pressure may differ between the right and left arm, and may result in a
different measurement value. Always use the same arm for measurements. If
the values between both arms differ substantially, check with your physician
on which arm to use for your measurements.
Battery Handling and Usage
• Disposal of used batteries should be carried out in accordance with local
regulations.

EN4
EN
•
3. Error Messages and Troubleshooting
If any of the below problems occur during measurement, check to make sure that no other electrical device is within 30 cm. If the problem persists, please refer to
the table below.
Display/Problem Possible Cause Solution
appears or the arm
cuff does not inflate.
The [START/STOP] button was pressed
while the arm cuff is not applied.
Press the [START/STOP] button again to turn the monitor off.
After inserting the air plug securely and applying the arm cuff
correctly, press the [START/STOP] button.
Air plug is not completely plugged
into the monitor.
Insert the air plug securely.
The arm cuff is not applied correctly. Apply the arm cuff correctly, then take another measurement.
Refer to section 7 of instruction manual
.
Air is leaking from the arm cuff. Replace the arm cuff to the new one. Refer to section 14 of
instruction manual
.
appears or a
measurement cannot
be completed after
the arm cuff inflates.
You move or talk during a measurement
and the arm cuff does not inflate
sufficiently.
Remain still and do not talk during a measurement. If “E2”
appears repeatedly, inflate the arm cuff manually until the
systolic pressure is 30 to 40mmHg above your previous
readings. Refer to section 13 of instruction manual
.
Due to the systolic pressure is above
210mmHg, a measurement cannot be
taken.
appears
The arm cuff is inflated exceeding the
maximum allowable pressure.
Do not touch the arm cuff and/or bend the air tube while
taking a measurement. If inflating the arm cuff manually, refer
to section 13 of instruction manual
.
appears
You move or talk during a measurement.
Vibrations disrupt a measurement.
Remain still and do not talk during a measurement.
appears
The pulse rate is not detected correctly. Apply the arm cuff correctly, then take another measurement.
Refer to section 7 of instruction manual
. Remain still and
sit correctly during a measurement.
If the “
” symbol continues to appear, we recommend you
to consult with your physician.
/ /
appears
does not flash during
a measurement

EN5
Display/Problem Possible Cause Solution
appears
Blood pressure measurements were
not taken correctly in an Afib mode
measurement.
Apply the arm cuff correctly, then take another measurement.
Refer to section 7 of instruction manual
. Remain still and
sit correctly during a measurement. Refer to section 8 of
instruction manual
.
appears
The monitor has malfunctioned. Press the [START/STOP] button again. If “Er” still appears,
contact your OMRON retail outlet or distributor.
appears
The montior cannot connect to a smart
device or transmit data correctly.
Follow the instructions shown in the “OMRON connect” app. If
the “Err” symbol still appears after checking the app, contact
your OMRON retail outlet or distributor.
flashes
The monitor is waiting for pairing with
the smart device.
Refer to section 5 of instruction manual
for pairing your
monitor with your smart device, or press [START/STOP] button
to cancel pairing and turn your monitor off.
flashes
The monitor is ready to transfer your
readings to the smart device.
Open the “OMRON connect” app to transfer your readings.
flashes
More than 80 readings are not
transferred.
Pair or transfer your readings to the “OMRON connect” app
so you can keep them in memory in the app, and this error
symbol disappears.
The date and time is not set.
appears
100 readings are not transferred.
flashes
Batteries are low. Replacing all 4 batteries with new ones is recommended.
Refer to section 4 of instruction manual
.
appears or the
monitor is turned off
unexpectedly during
a measurement
Batteries are depleted. Immediately replace all 4 batteries with new ones. Refer to
section 4 of instruction manual
.
Nothing appears on the display
of the monitor.
Battery polarities are not properly
aligned.
Check the battery installation for proper placement. Refer to
section 4 of instruction manual
.
Readings appear too high or
too low.
Blood pressure varies constantly. Many factors including stress, time of day, and/or how you apply the arm
cuff, may affect your blood pressure. Review sections 2 of instruction manual
.
Any other communication
issue occurs.
Follow the instructions shown in the smart device, or visit the “Help” section in the “OMRON connect” app
for further help. If the problem still persists, contact your OMRON retail outlet or distributor.

EN6
EN
Display/Problem Possible Cause Solution
Any other problem occurs.
Press the [START/STOP] button to turn the monitor off, then press it again to take a measurement. If the
problem continues, remove all batteries and wait for 30 seconds. Then re-install batteries. If the problem
still persists, contact your OMRON retail outlet or distributor.
Troubleshooting for Afib indicator function:
What is different between the
Afib indicator function and
ECG?
The Afib indicator function and ECG use completely different technologies. An ECG measures the electrical
activity of the heart and can be used to diagnose Afib. The Afib indicator function detects irregular
heartbeat and can suggest the possibility of Afib with a sensitivity of 95.5% and specificity of 93.8%.
Refer
to section 11 for details.
If the “ ” symbol does not
appear, it means there is no
possibility of Afib?
Even if the “ ” symbol does not appear, there is still a possibility of Afib.
Should I consult with my
physician if the “
” symbol
appears?
We recommend you to consult with your physician because there is a possibility of Afib. However, the “
”
symbol may be displayed for other reasons, such as other heart arrhythmias.
What is different between
Afib indicator function and
irregular heart beat function?
The irregular heartbeat function detects irregularities in the pulse waves in one measurement. The Afib
indicator function suggests the possibility of Afib when blood pressure is measured 3 consecutive times.
What should I do if the “
”
symbol sometimes appears?
Afib does not always have symptoms. We recommend you to consult with and follow the directions of
your physician.
I have been diagnosed with
Afib by the physician, but the
“
” symbol does not appear.
Afib may not occur at the time of specific blood pressure measurements. We recommend you to consult
with your physician regularly.
Is the blood pressure reading
reliable when the “
”
symbol appears?
Afib or an irregular heartbeat can influence your blood pressure measurements and make it difficult to get
an accurate reading. Repeated measurements may be required to overcome variabilities.* In Afib mode,
the blood pressure measurement is taken 3 times, and the average is displayed. The monitor will indicate
an error message (E5/E6) if the influence of the irregular heartbeat is too severe to give a measurement
result. If this occurs repeatedly, we recommend that you consult with your physician.
* Prof. Roland Asmar et al. European Society of Hypertension Recommendations for Conventional, Ambulatory and Home Blood Pressure Measurement

EN7
4. Limited Warranty
Thank you for buying an OMRON product. This product is constructed of high
quality materials and great care has been taken in its manufacturing. It is
designed to give you every satisfaction, provided that it is properly operated
and maintained as described in the instruction manual.
This product is warranted by OMRON for a period of 3 years after the date of
purchase. The proper construction, workmanship and materials of this product
is warranted by OMRON. During this period of warranty OMRON will, without
charge for labour or parts, repair or replace the defect product or any defective
parts.
The warranty does not cover any of the following:
A. Transport costs and risks of transport.
B. Costs for repairs and / or defects resulting from repairs done by unauthorised
persons.
C. Periodic check-ups and maintenance.
D. Failure or wear of optional parts or other attachments other than the main
device itself, unless explicitly warranted above.
E. Costs arising due to non-acceptance of a claim (those will be charged for).
F. Damages of any kind including personal caused accidentally or from misuse.
G. Calibration service is not included within the warranty.
H. Optional parts have a one (1) year warranty from date of purchase. Optional
parts include, but are not limited to the following items: cuff and cuff tube.
Should warranty service be required please apply to the dealer whom the
product was purchased from or an authorised OMRON distributor. For the
address refer to the product packaging / literature or to your specialised
retailer. If you have difficulties in finding OMRON customer services, contact us
for information:
www.omron-healthcare.com
Repair or replacement under the warranty does not give rise to any extension
or renewal of the warranty period.
The warranty will be granted only if the complete product is returned together
with the original invoice / cash ticket issued to the consumer by the retailer.
5. Maintenance
5.1 Maintenance
To protect your monitor from damage, follow the directions below:
Changes or modifications not approved by the manufacturer will void the user
warranty.
Caution
DO NOT disassemble or attempt to repair this monitor or other components.
This may cause an inaccurate reading.
5.2 Storage
• Keep your monitor in the storage case when not in use.
1. Remove the arm cuff from the monitor.
Caution
To unplug the air plug, pull on the plastic air plug at the base of the tube, not
the tube itself.
2. Gently fold the air tube into the arm cuff. Note: Do not bend or crease the
air tube excessively.
3. Place your monitor and other components in the storage case.
• Store your monitor and other components in a clean, safe location.
• Do not store your monitor and other components:
• If your monitor and other components are wet.
• In locations exposed to extreme temperatures, humidity, direct sunlight,
dust or corrosive vapors such as bleach.
• In locations exposed to vibrations or shocks.
• To protect your monitor during storage, an optional LCD cover is available as
accessory. Refer to section 15 of Instruction Manual
.
5.3 Cleaning
• Do not use any abrasive or volatile cleaners.
• Use a soft dry cloth or a soft cloth moistened with mild (neutral) detergent to
clean your monitor and arm cuff, and then wipe them with a dry cloth.
• Do not wash or immerse your monitor and arm cuff or other components in
water.
• Do not use gasoline, thinners or similar solvents to clean your monitor and
arm cuff or other components.
5.4 Calibration and Service
• The accuracy of this blood pressure monitor has been carefully tested and is
designed for a long service life.
• It is generally recommended to have the unit inspected every two years to
ensure correct functioning and accuracy. Please consult your authorised
OMRON dealer or the OMRON Customer Service at the address given on the
packaging or attached literature.

EN8
EN
6. Specications
Product Category Electronic Sphygmomanometers
Product description Automatic Upper Arm Blood Pressure
Monitor
Model (Code) M7 Intelli IT (HEM-7361T-EBK) /
X7 Smart (HEM-7361T-ESL)
Display LCD digital display
Cuff pressure range 0 to 299mmHg
Blood pressure
measurement range
SYS: 60 to 260mmHg
DIA: 40 to 215mmHg
Pulse measurement range
40 to 180 beats / min.
Accuracy Pressure: ±3mmHg
Pulse: ±5% of display reading
Inflation Automatic by electric pump
Deflation Automatic pressure release valve
Measurement method Oscillometric method
Transmission method Bluetooth
®
Low Energy
Wireless communication Frequency range: 2.4GHz (2400 -
2483.5 MHz) / Modulation: GFSK
Effective radiated power: <20dBm
Operation mode Continuous operation
IP classification Monitor: IP20
Optional AC adapter: IP21
Rating DC6V 4.0W
Power source 4 “AA” batteries 1.5 V or optional
AC adapter (INPUT AC 100 - 240V
50/60Hz 0.12 - 0.065A)
Battery life Approximately 1000 measurements
(using new alkaline batteries)
The number of times may decrease when
using Afib mode because one Afib indication
consists of 3 regular measurements.
Durable period (Service
life)
Monitor: 5 years / Cuff: 5 years /
Optional AC adapter: 5 years
Operating conditions +10 to +40°C / 15 to 90% RH
(non-condensing) / 800 to 1060hPa
Storage / Transport
conditions
-20 to +60°C / 10 to 90% RH
(non-condensing)
Weight Monitor: approximately 460g
(notincluding batteries)
Arm cuff: approximately 163g
Dimensions
(approximately value)
Monitor: 191mm (W) × 85mm (H) ×
120mm (L) / Arm cuff:145mm ×
532mm (air tube: 750mm)
Cuff circumference
applicable to the monitor
220 to420 mm
Memory Stores up to 100 readings per user
Contents Monitor, arm cuff (HEM-FL31), 4 “AA”
batteries, Instruction Manual
and
, setup instructions, storage case
Protection against
electric shock
Internally powered ME equipment
(When using only batteries)
Class II ME equipment (Optional AC
adapter)
Applied part Type BF (arm cuff)
Note
• These specifications are subject to change without notice.
• This monitor is clinically investigated according to the requirements of
ISO81060-2:2013. In the clinical validation study, K5 was used on 85 subjects
for determination of diastolic blood pressure.
• This device has been validated for use on pregnant and pre-eclampsia patients
according to the Modified European Society of Hypertension Protocol*.
• This device has been validated for use on diabetic (TypeII) population**.

EN9
• IP classification is degrees of protection provided by enclosures in
accordance with IEC60529. This monitor and optional AC adapter are
protected against solid foreign objects of 12.5mm diameter and greater
such as a finger. The optional AC adapter is protected against vertically
falling water drops which may cause issues during a normal operation.
* Topouchian J et al. Vascular Health and Risk Management 2018:14 189–197
** Chahine M.N. et al. Medical Devices: Evidence and Research 2018:11 11–20
About a wireless communication interference
This product operates in an unlicensed ISM band at 2.4GHz. In the event
this product is used near other wireless devices such as microwave and
wireless LAN, which operate on the same frequency band as this product,
there is a possibility that interference may occur. If interference occurs, stop
the operation of the other devices or relocate this product away from other
wireless devices before attempting to use it.
7. Correct Disposal of This Product (Waste
Electrical & Electronic Equipment)
This marking shown on the product or its literature, indicates that
it should not be disposed of, with other household wastes at the
end of its working life.
To prevent possible harm to the environment or human health
from uncontrolled waste disposal, please separate this product
from other types of wastes and recycle it responsibly to promote
the sustainable reuse of material resources.
Household users should contact either the retailer where they purchased this
product, or their local government office, for details of where and how they can
return this item for environmentally safe recycling.
Business users should contact their supplier and check the terms and
conditions of the purchase contract. This product should not be mixed with
other commercial waste for disposal.
8. Important Information regarding
Electromagnetic Compatibility (EMC)
HEM-7361T-EBK/ESL conforms to the EN60601-1-2:2015 Electromagnetic
Compatibility (EMC) standard.
Further documentation in accordance with this EMC standard is available at
OMRON HEALTHCARE EUROPE at the address mentioned in this instruction
manual or at: www.omron-healthcare.com.
9. Guidance and Manufacturer’s Declaration
• This blood pressure monitor is designed according to the European Standard
EN1060, Non-invasive sphygmomanometers Part 1: General Requirements
and Part 3: Supplementary requirements for electromechanical blood
pressure measuring systems.
• Hereby, OMRON HEALTHCARE Co., Ltd., declares that the radio equipments
type HEM-7361T-EBK/ESL is in compliance with Directive 2014/53/EU.
• The full text of the EU declaration of conformity is available at the following
internet address: www.omron-healthcare.com
• This OMRON product is produced under the strict quality system of OMRON
HEALTHCARE Co., Ltd., Japan. The Core component for OMRON blood
pressure monitors, which is the Pressure Sensor, is produced in Japan.
• Please report to the manufacturer and the competent authority of the
Member State in which you are established about any serious incident that
has occurred in relation to this device.
10. How to Calculate Weekly Averages
Morning Weekly Average Calculation
This is the average for the measurements taken during the morning (4:00 - 9:59)
between Sunday and Saturday. The 2 or 3 readings taken within the first
10minute timeframe in the morning between 4:00 - 9:59 will be used to
calculate the morning average for each day.
Evening Weekly Average Calculation
This is the average for the measurements taken during the evening (19:00 - 1:59)
between Sunday and Saturday. The 2 or 3 readings taken within the last
10minute timeframe in the evening between 19:00 - 1:59 will be used to
calculate the evening average for each day.
Within 10 min.
Measurements
in the morning
Measurements
in the morning
Within 10 min.

EN10
EN
11. Useful Information
What is Blood Pressure?
Blood pressure is a measure of the force of blood flowing against the walls of
the arteries. Arterial blood pressure is constantly changing during the course of
the heart’s cycle.
The highest pressure in the cycle is called the Systolic Blood Pressure; the lowest
is the Diastolic Blood Pressure. Both pressures, the Systolic and Diastolic, are
necessary to enable a physician to evaluate the status of a patient’s blood pressure.
What is Arrhythmia?
Arrhythmia is a condition where the heartbeat rhythm is abnormal due to flaws in
the bio-electrical system that drives the heartbeat. Typical symptoms are skipped
heartbeats, premature contraction, an abnormally rapid (tachycardia) or slow
(bradycardia) pulse.
What is Afib?
Atrial fibrillation (also called AFib or AF) is a quivering or irregular heartbeat
(arrhythmia) that can lead to blood clots, stroke, heart failure and other heart-
related complications. During atrial fibrillation, the heart’s two upper chambers (the
atria) beat chaotically and irregularly — out of coordination with the two lower
chambers (the ventricles) of the heart. Episodes of atrial fibrillation can come and
go, or you may develop atrial fibrillation that doesn’t go away and may require
treatment.
Afib indicator function detects the possibility of Afib with an accuracy of 94.2%
(with sensitivity of 95.5% and specificity of 93.8%) as demonstrated in the study*
with Single-lead ECG as reference measurement.
*M. Ishizawa, T. Noma, T. Minamino et al., Multiple measurements with
automated blood pressure monitor can detect atrial fibrillation with high
sensitivity and specificity in general cardiac patients, ESC Congress 2018

SD1
Symbols Description
FR
Description des symboles
NL
Beschrijving van symbolen
DE
Beschreibung der Symbole
RU
IT
Descrizione dei simboli
TR
Simgelerin Açıklaması
ES
Descripción de los símbolos
AR
Applied part - Type BF Degree of protection against
electric shock (leakage current)
FR
Partie appliquée - Type BF
Degré de protection contre
les chocs électriques
(courant de fuite)
DE
Anwendungsteil– Typ BF
Schutz vor Stromschlägen
(Ableitstrom)
IT
Parti applicate - Tipo BF
Livello di protezione contro
le folgorazioni (corrente di
dispersione)
ES
Partes en contacto: Tipo
BF Grado de protección
contra descargas eléctricas
(corriente de fuga)
NL
Toegepast
onderdeel - Type BF-
beschermingsgraad tegen
elektrische schokken
(lekstroom)
RU
- BF
( )
TR
Uygulanan parça - Tip BF
Elektrik çarpmasına karşı
koruma derecesi (kaçak
akım)
Class II equipment. Protection against electric shock
FR
Équipement de classe II.
Protection contre les chocs
électriques
DE
Gerät der Klasse II. Schutz
vor Stromschlägen
IT
Apparecchiatura di
ClasseII. Protezione contro
le folgorazioni
ES
Equipo de Clase II.
Protección contra
descargas eléctricas
NL
Apparatuur van KlasseII.
Bescherming tegen
elektrische schokken
RU
II.
TR
Sınıf II ekpman. Elektrk
çarpmasına karşı koruma
IP XX
Ingress protection degree provided by IEC 60529
FR
Degré de protection selon
CEI60529
DE
Grad des Eindringschutzes
gemäß IEC60529
IT
Livello di protezione IP in
base a IEC 60529
ES
Grado de protección según
la norma internacional
IEC60529
NL
Beschermingsklasse
volgens IEC60529
RU
,
,
IEC 60529
TR
Su grmesne karşı koruma
dereces IEC 60529
tarafından verlmştr
CE Marking
FR
Marquage CE
DE
CE-Kennzeichnung
IT
Contrassegno CE
ES
Marcado CE
NL
CE-merkteken
RU
TR
CE İşaret
Serial number
FR
Numéro de série
DE
Seriennummer
IT
Numero di serie
ES
Número de serie
NL
Serienummer
RU
TR
Ser numarası
LOT number
FR
Numéro de LOT
DE
LOT-Nummer
IT
Numero di lotto
ES
Número de lote
NL
Partijnummer
RU
TR
Parti numarası
Medical device
FR
Dispositif médical
DE
Medizinprodukt
IT
Dispositivo medico
ES
Producto sanitario
NL
Medisch apparaat
RU
TR
Tıbbi cihaz
BF
AR
AR
AR
)CE
AR
AR
AR
II
AR

SD2
Indicates the manufacturer’s catalogue number
FR
Indique le numéro de
catalogue du fabricant
DE
Angabe der Hersteller-
Katalognummer
IT
Indica il numero di
catalogo del produttore
ES
Indica el número de
catálogo del fabricante
NL
Geeft het
catalogusnummer van de
fabrikant aan
RU
TR
Üretcnn katalog
numarasını belrtr
Temperature limitation
FR
Limitation de température
DE
Temperaturbegrenzung
IT
Limite di temperatura
ES
Limitación de la
temperatura
NL
Temperatuurbegrenzing
RU
TR
Sıcaklık sınırlaması
Humidity limitation
FR
Limitation d’humidité
DE
Luftfeuchtigkeits-
begrenzung
IT
Limite di umidità
ES
Limitación de la humedad
NL
Vochtigheidsbegrenzing
RU
TR
Nem sınırlaması
Atmospheric pressure limitation
FR
Limitation de pression
atmosphérique
DE
Luftdruckbegrenzung
IT
Limite di pressione
atmosferica
ES
Limitación de la presión
atmosférica
NL
Luchtdrukbegrenzing
RU
TR
Atmosferk basınç
sınırlaması
Indication of connector polarity
FR
Indication de la polarité
des connecteurs
DE
Anzeige der
Steckerpolarität
IT
Indicazione della polarità
dei connettori
ES
Indicación de la polaridad
del conector
NL
Indicatie van polariteit van
aansluiting
RU
TR
Bağlantı polarite
göstergesi
For indoor use only
FR
Pour un usage à l’intérieur
uniquement
DE
Nur für die Nutzung in
Innenbereichen
IT
Solo per uso in interni
ES
Para uso solo en interiores
NL
Alleen voor gebruik
binnenshuis
RU
TR
Sadece ç mekanda
kullanım çn
OMRON’s trademarked technology for blood
pressure measurement
FR
Technologie brevetée
OMRON pour la mesure de
la pression artérielle
DE
Markenrechtlich
geschützte Technologie
von OMRON zur
Blutdruckmessung
IT
Tecnologia brevettata
OMRON per la misurazione
della pressione arteriosa
ES
La tecnología de OMRON
para medir la presión
arterial
NL
Technologie voor
bloeddrukmeting onder
handelsmerk van OMRON
RU
OMRON
TR
OMRON'un kan basıncı
ölçümü çn tcar markalı
teknolojsdr
AR
AR
AR
OMRON
AR
AR
AR
AR

SD3
Identifier of cuffs compatible for the device
FR
Identificateur des brassards
compatibles avec l’appareil
DE
Kennzeichnung der mit
dem Gerät kompatiblen
Manschetten
IT
Identifica i bracciali
compatibili con il
dispositivo
ES
Identificador para
manguitos compatibles
con el dispositivo
NL
Identificatie van
manchetten die
compatibel zijn met het
apparaat
RU
,
TR
Chaz le uyumlu
kollukların tanıtım şaret
Marker on the cuff to be positioned above the
artery
FR
Repère sur le brassard, à
positionner au-dessus de
l’artère
DE
Markierung auf der
Manschette, die oberhalb
der Arterie liegen muss
IT
Contrassegno sul bracciale
da posizionare al di sopra
dell’arteria
ES
La marca del manguito
debe colocarse sobre la
arteria
NL
Markering op de manchet
die boven de slagader
moet worden geplaatst
RU
TR
Kolluk üzerndek şaretn
konumu artern üzerne
gelmeldr
,
Manufacturer’s quality control mark
FR
Marque de contrôle de la
qualité du fabricant
DE
Qualitätskontrollzeichen
des Herstellers
IT
Contrassegno controllo
qualità del produttore
ES
Marca del control de
calidad del fabricante
NL
Symbool voor
kwaliteitscontrole van
fabrikant
RU
TR
Üretcnn kalte kontrol
şaret
Not made with natural rubber latex
FR
Ne contient pas de latex de
caoutchouc naturel
DE
Enthält kein Naturlatex
IT
Non contiene lattice di
gomma naturale
ES
No contiene látex de
caucho natural
NL
Bevat geen
natuurrubberlatex
RU
TR
Doğal kauçuk lateksten
üretlmemştr
Arm circumference
FR
Circonférence du bras
DE
Armumfang
IT
Circonferenza del braccio
ES
Perímetro de brazo
NL
Armomtrek
RU
TR
Kol çevres
Necessity for the user to consult this instruction manual
FR
L’utilisateur doit consulter
le mode d’emploi
DE
Der Benutzer muss diese
Gebrauchsanweisung
lesen
IT
L’utente deve consultare
il presente manuale di
istruzioni
ES
Es necesario que el usuario
consulte este manual de
instrucciones
NL
De gebruiker dient deze
gebruiksaanwijzing te
raadplegen
RU
TR
Kullanıcı, bu kullanım
kılavuzuna başvurmalıdır
AR
AR
AR
AR
AR
AR

SD4
Need for the user to follow this instruction manual
thoroughly for your safety.
FR
L’utilisateur doit suivre
attentivement ce mode
d’emploi pour votre
sécurité.
DE
Damit die Sicherheit
gewährleistet ist, muss
der Benutzer diese
Gebrauchsanweisung
sorgfältig befolgen.
IT
Per la propria sicurezza,
l’utente deve seguire
attentamente il presente
manuale di istruzioni.
ES
Es necesario que el usuario
siga rigurosamente este
manual de instrucciones
para su seguridad.
NL
Voor de eigen veiligheid
dient de gebruiker zich
zorgvuldig aan deze
gebruiksaanwijzing te
houden.
RU
.
TR
Güvenlk açısından
kullanıcının bu kullanım
kılavuzuna dkkatle
uyması gerekr.
Direct current
FR
Courant continu
DE
Gleichstrom
IT
Corrente diretta
ES
Corriente directa
NL
Gelijkstroom
RU
TR
Doğru akım
Alternating current
FR
Courant alternatif
DE
Wechselstrom
IT
Corrente alternata
ES
Corriente alterna
NL
Wisselstroom
RU
TR
Alternatf akım
Date of manufacture
FR
Date de fabrication
DE
Herstellungsdatum
IT
Data di fabbricazione
ES
Fecha de fabricación
NL
Productiedatum
RU
TR
Üretm tarh
Prohibited action
FR
Action interdite
DE
Verbotene Aktion
IT
Operazione proibita
ES
Acción prohibida
NL
Verboden handeling
RU
TR
Yasaklanmış eylem
AR
AR
AR
AR
AR

SD5
To indicate generally elevated, potentially hazardous, levels of non-ionizing radiation, or to indicate equipment or systems.
e.g. in the medical electrical area that include RF transmitters or that intentionally apply RF electromagnetic energy for
diagnosis or treatment.
FR
Pour indiquer des niveaux généralement élevés, potentiellement
dangereux, de rayonnement non ionisant, ou pour indiquer
l’équipement ou les systèmes, par exemple dans le domaine de
l’électricité médicale qui comprennent des émetteurs RF ou qui
utilisent intentionnellement l’énergie électromagnétique RF pour le
diagnostic ou le traitement.
DE
Als Hinweis auf allgemein erhöhte, potenziell gefährliche Stufen
nicht-ionisierender Strahlung oder als Hinweis auf Geräte oder
Systeme zum Beispiel im medizinisch-elektrischen Bereich, etwa HF-
Übertragungsgeräte, bzw. auf solche, die elektromagnetische HF-
Strahlung zur Diagnose oder Behandlung verwenden.
IT
Indica livelli generalmente elevati, potenzialmente pericolosi, di
radiazioni non ionizzanti oppure indica apparecchiature o sistemi
(ad esempio per le aree elettromedicali in cui sono presenti
trasmettitori RF o in cui viene intenzionalmente applicata energia
elettromagnetica a radiofrequenza per la diagnosi o il trattamento).
ES
Para indicar niveles de radiación no ionizante generalmente
elevados y potencialmente peligrosos, o bien para indicar equipos
o sistemas, como los usados en el ámbito electro médico, que
incorporen transmisores de radiofrecuencia o que apliquen energía
electromagnética de radiofrecuencia intencionadamente para
diagnósticos o tratamientos.
NL
Geeft in het algemeen verhoogde, potentieel gevaarlijke niveaus aan
van niet-ioniserende straling of duidt op apparatuur of systemen,
bijvoorbeeld in de medische elektrische omgeving, die RF-zenders
bevatten of die opzettelijk elektromagnetische RF-energie toepassen
voor diagnose of behandeling.
RU
(,
),
,
.
TR
Genellkle yüksek ve zararlı olablecek yonlaşmayan radyasyon
sevyelern belrtr veya RF vercler çeren veya tanı ya da tedav
amacıyla blnçl olarak RF elektromanyetk enerj uygulayan (örneğn
medkal elektrk alanında bulunan) ekpman ve sstemler belrtr.
AR

SD6
The Bluetooth® word mark and logos are registered trademarks owned by the Bluetooth SIG, Inc. and any use of such marks by OMRON HEALTHCARE Co., Ltd. is
under license. Other trademarks and trade names are those of their respective owners. App Store is a service mark of Apple Inc., registered in the U.S and other
countries. Google Play logo are trademarks of Google LLC.
FR
La marque verbale et les logos Bluetooth® sont des marques déposées
détenues par Bluetooth SIG, Inc. et l’utilisation de ces marques par OMRON
HEALTHCARE Co., Ltd. se fait sous licence. Les autres marques commerciales
et noms de marque sont ceux de leurs détenteurs respectifs. AppStore est
une marque de service d’Apple Inc., déposées aux États-Unis et dans d’autres
pays. Le logo GooglePlay est une marque commerciale de GoogleLLC.
DE
Die Bluetooth®-Wortmarke und -Logos sind eingetragene Marken
der Bluetooth SIG, Inc. und die Verwendung solcher Marken durch
OMRON HEALTHCARE Co., Ltd. erfolgt in Lizenz. Andere Marken und
Markennamen gehören ihren jeweiligen Eigentümern. AppStore ist eine
Dienstleistungsmarke der AppleInc., die in den USA und anderen Ländern
eingetragen ist. Das Google Play-Logo ist eine Marke der Google LLC.
IT
Il marchio e i logotipi Bluetooth® sono marchi commerciali registrati di
Bluetooth SIG, Inc. e l’utilizzo di tali marchi da parte di OMRON HEALTHCARE
Co., Ltd. è stato concesso in licenza. Gli altri marchi e nomi commerciali sono
di proprietà dei rispettivi titolari. App Store è un marchio commerciale di
Apple Inc., registrato negli Stati Uniti e in altri Paesi. Il logo Google Play è un
marchio commerciale di Google LLC.
ES
El nombre y los logotipos de Bluetooth® son marcas registradas de
Bluetooth SIG, Inc. y cualquier uso de dichas marcas hecho por OMRON
HEALTHCARE Co., Ltd. se ha llevado a cabo con su licencia correspondiente.
Otras marcas registradas también pertenecen a sus respectivos propietarios.
App Store es una marca de servicio registrada de Apple Inc. en EE. UU. y en
otros países. El logotipo de Google Play es una marca comercial de Google
LLC.
NL
Het woordmerk en de logo’s van Bluetooth® zijn gedeponeerde
handelsmerken van Bluetooth SIG, Inc. en enig gebruik hiervan door
OMRON HEALTHCARE Co., Ltd. geschiedt onder licentie. Overige
handelsmerken en handelsnamen zijn van hun respectievelijke eigenaren.
App Store is een servicemerk van Apple Inc. en gedeponeerd in de V.S. en in
andere landen. Het Google Play-logo is een handelsmerk van Google LLC.
RU
Bluetooth®
, Bluetooth
SIG, Inc., OMRON
HEALTHCARE Co., Ltd. .
. App Store Apple Inc.,
. Google Play
Google LLC.
TR
Bluetooth® marka adı ve logoları, Bluetooth SIG Inc. kuruluşunun tescilli
ticari markalarıdır ve OMRON HEALTHCARE Co., Ltd. bu markaları lisans
kapsamında kullanmaktadır. Diğer ticari markalar ve ticari isimler, ilgili
sahiplerine aittir. App Store, Apple Inc. firmasının ABD ve diğer ülkelerde
tescilli hizmet markasıdır. Google Play logosu, GoogleLLC firmasının ticari
markasıdır.
Bluetooth
®
AR
.
Play

Issue Date:
2019-09-04
Date de publication:
Ausgabedatum:
Data di pubblicazione:
Fecha de publicación:
Uitgiftedatum
:
Teslim Tarihi:
IM1-HEM-7361T-E-02-09/2019
2895864-6B

Automatic Upper Arm
Blood Pressure Monitor
M7 Intelli IT (HEM-7361T-EBK)
X7 Smart (HEM-7361T-ESL)
Instruction Manual
2
Read Instruction manual and before use.
FR
Lire le mode d’emploi et vant l’utilisation.
DE
Lesen Sie vor der Verwendung Gebrauchsanweisung und .
IT
Leggere il manuale di istruzioni e prima dell’uso.
ES
Lea el manual de instrucciones y antes del uso.
NL
Lees gebruiksaanwijzing en voor gebruik.
RU
.
TR
Kullanmadan önce, kullanım kılavuzu ve 'yi okuyun.
AR

1
Package Contents...........................................1
FR
Contenu de l’emballage
NL
Inhoud van de verpakking
DE
Packungsinhalt
RU
IT
Contenuto della confezione
TR
Paketin İçindekiler
ES
Contenido del envase
AR
2
Preparing for a Measurement........................4
FR
Préparation pour une prise
de mesure
NL
Een meting voorbereiden
DE
Vorbereiten einer Messung
RU
IT
Preparazione per la
misurazione
TR
Ölçüm Hazırlığı
ES
Preparación para una
medición
AR
3
Downloading the "OMRON connect" App.....5
FR
Téléchargement de
l’application «OMRON
connect»
NL
De app “OMRON connect“
downloaden
DE
Herunterladen der App
„OMRON connect“
RU
«OMRON connect»
IT
Download dell'app “OMRON
connect”
TR
"OMRON Connect"
Uygulamasını İndirme
ES
Descarga de la aplicación
“OMRON connect”
AR
4
Inserting Batteries..........................................6
FR
Mise en place des piles
NL
De batterijen plaatsen
DE
Einlegen von Batterien
RU
IT
Inserimento delle batterie
TR
Pilleri Takma
ES
Introducción de las pilas
AR
5
Pairing Your Smart Device..............................7
FR
Jumelage de votre appareil
intelligent
NL
Uw smartapparaat koppelen
DE
Koppeln mit Smartphone
oder Tablet
RU
-
IT
Associazione del dispositivo
smart
TR
Akıllı Cihazınızın
Eşleştirilmesi
ES
Sincronización con un
dispositivo inteligente
AR
6
Setting Date and Time Manually....................8
FR
Réglage manuel de la date
et de l’heure
NL
Datum en tijd handmatig
instellen
DE
Manuelles Einstellen von
Datum und Uhrzeit
RU
IT
Impostazione manuale di
data e ora
TR
Tarih ve Saatin Manuel
Olarak Ayarlanması
ES
Ajuste manual de la fecha y
la hora
AR
7
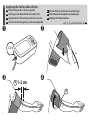
Applying the Cuff on the Left Arm.................9
FR
Pose du brassard sur le bras
gauche
NL
De manchet op de linkerarm
aanbrengen
DE
Anbringen der Manschette
am linken Arm
RU
IT
Applicazione del bracciale
sul braccio sinistro
TR
Kolluğun Sol Kola Takılması
ES
Colocación del manguito en
el brazo izquierdo
AR
8
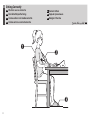
Sitting Correctly..............................................11
FR
Position assise correcte
NL
Correct zitten
DE
Korrekte Körperhaltung
RU
IT
Come sedersi nel modo
corretto
TR
Düzgün Oturma
ES
Cómo sentarse
correctamente
AR
1
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
-
 45
45
-
 46
46
-
 47
47
-
 48
48
-
 49
49
-
 50
50
Omron Healthcare HEM-7361T-EBK Manuale utente
- Categoria
- Unità di pressione sanguigna
- Tipo
- Manuale utente
in altre lingue
Documenti correlati
-
Omron Healthcare X7 Smart - HEM-7361T-ESL Manuale del proprietario
-
Omron Healthcare HEM-7361T-EBK Manuale utente
-
Omron Healthcare HEM-7360-E Manuale utente
-
Omron Healthcare HEM-7361T-EBK Manuale utente
-
Omron Healthcare HEM-7154-E Manuale utente
-
Omron Healthcare HEM-7155-E Manuale utente
-
Omron Healthcare X6 Comfort Manuale utente
-
Omron Healthcare HEM-7155T-EBK Manuale utente
-
Omron Healthcare M4 Intelli IT - HEM-7155T-EBK Manuale del proprietario
-
Omron Healthcare M2 Manuale utente
Altri documenti
-
Omron HEM-7143T2-ESL Manuale utente
-
Omron M7 Intelli IT Manuale utente
-
Omron HEM-7155T-ESL Manuale utente
-
 AND UA-767NFC Manuale utente
AND UA-767NFC Manuale utente
-
A&D UA-767 Plus BT-Ci Manuale utente
-
Gima 49902 Manuale del proprietario
-
 Nissei DSK-1011 Istruzioni per l'uso
Nissei DSK-1011 Istruzioni per l'uso
-
Omron RX-3 RX-3 Manuale utente
-
Panasonic EWBU60W800 Istruzioni per l'uso
-
iHealth Clear BPM1 Manuale del proprietario



















































