
Molift Air Tilt
BM14099 Rev. D 2021-05-18
Gebrauchsanweisung - Deutsch
Handleiding - Nederlands
Manual d`utilisation - Francais
Manual utente - Italiano
Manual de usuarioo - Español
User manual - English
Bruksanvisning - Svenska
Brukermanual - Norsk
Brugsvejledning - Dansk
Käyttöohje - Suomi


Contents
User manual - English - 1
Bruksanvisning - Svenska - 7
Brukermanual - Norsk - 13
Brugsvejledning - Dansk - 19
Käyttöohje - Suomi - 25
Gebrauchsanweisung - Deutsch - 31
Handleiding - Nederlands - 37
Manual d`utilisation - Francais - 43
Manual utente - Italiano - 49
Manual de usuarioo - Español - 55

Molift Air Tilt / www.etac.com
1
Important
This User Manual contains important safety instructions and
information regarding the use of the sling and accessories.
In this manual the client is the person being lifted.
The caregiver is the person operating the hoist.
This symbol indicates important information
related to safety. Follow these instructions
carefully.
Read User Manual before use!
It is important to fully understand the con-
tent of the user manual before attempting to
use the equipment.
Visit www.etac.com for download of documentation to ensure
you have the latest version.
General
Conditions for Use
Lift and transfer of a client will always pose a certain risk and
only trained personnel are allowed to use the equipment and
accessories covered by this user manual. The rail system must
be installed by certified personnel in accordance with applicable
installation instructions.
Modifications and use of components made by other manufacturers.
We recommend only using Molift components and spare parts.
Declaration of conformity is not valid and Etac is not responsible
for warranty if any modifications are made to the product. Etac
shall not be liable for faults or accidents that can occur when
using components made by other manufacturers.
The hoist is not intended to be operated by the client being lifted.
If a hoist is to be used by a client living on their own, then some
form of communication device shall be installed in the area of use
of the hoist so that in the event of an emergency the client is able
to summon assistance. This may, for example, be the fitting of an
alarm system or the supply of a conveniently placed telephone,
etc.
Only certified personnel are allowed to open
hoist or accessories to perform service or
repair. Risk of injury from rotating parts and
electric shock.
Warranty notice
Two-year warranty against defects in work-
manship and materials of our products.
One-year warranty for batteries.
Please refer to www.etac.com for terms and
conditions.
CE declaration
The product, and its related accesso-
ries, described in this instruction for
use (IFU), is in compliance with the
regulation (EU) 2017/745 of 5. April
2017 – as a medical device, risk class I.
The product has been tested and ap-
proved by a third party according to
standards IEC 60601-1, IEC 60601-1-2
and EN/ISO 10535:2006.
Any serious incident that occurs in
relation to the device should be
reported to the manufacturer and the
competent authority of the Member
State in which the user and/or patient
is established.

Molift Air Tilt / www.etac.com
2
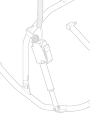
Molift Air Tilt
1
2
3
5
4
Components:
1. Molift Air 350 Tilt
2. Quick release pin
3. Molift Air Tilt Hand control
4. Cable for connection to Molift Air 350 Tilt
5. Molift Air Tilt Sling bar
About Molift Air Tilt
Molift Air Tilt Sling bar is an accessory to Molift Air 350 Tilt ceiling
hoist as an alternative to standard sling bars. The Molift Air Tilt
has clip studs for clip sling attachment (accessory) and with the
adjustable sling bar, supported by an electric actuator, it allows
the caregiver to control and adjust the position of the patient,
e.g. from upright to reclined.
Product identification
Product label
1. Product label, 2. Product label, 3. Actuator label (Linak),
4. Serial number label
Warning labels and Symbols
Symbols used on the product, explained in more detail:
ENSVNODAFIDENLFRITES
Molift Air 350 Tilt
Gross weight 361kg / 797lbs
SWL 350kg / 772lbs
24V SLA, IP24
Duty cycle 10%
Molift Air Tilt Sling bar
Gross weight 279,5kg / 616lbs
SWL 272kg / 600lbs
24V SLA, IP24
Duty cycle 10%
Compliance to all relevant
EC directives
IEC 60417-5172:
Class II equipment
Refer to user manual Operating instructions
Indoor Use only
Manufacturer
Max user weight
YYYY-MM-DD
Date of manufacture
Do not dispose in general
waste
WEEE Directive
2002/96/EC
Catalogue number
Low battery indicator (hand
control)
Serial number
China Pollution control
mark (also indicates
recyclability) China RoHS
legislation
Medical Device
Australian Regulatory
Compliance Mark –
EMC, Electrical safety (RCM)

Molift Air Tilt / www.etac.com
3
Working pause ratio/Duty Cycle.
Duty Cycle 10%. (Intermittence according to standard ISO-EN
10535)
Technical data
Safe Working Load (SWL)
272 kg (600 lbs)
Weight of unit (excluding hand control and cables):
7,3 kg (16 lbs)
Actuator speed
12,6-10,4mm/second (0,49-0,40 inches/second)
Protection class:
Hand control IP24
Actuator IPX4
Operating forces button
Buttons on handset: 3.4 N
Material:
Steel, Plastic
Expected Lifetime:
Expected lifetime of 30 000 cycles SWL or 10 years.
Dimensions:
L x W x H (Length, Width)
66 x 58 x 50-79 cm
(26 x 22,8 x 19,7-33,5 inches)
Tilting Range:
75 degrees
75°
47cm
50-79cm
58cm
58cm
Electronics
Transport and Storage
The hoist can be stored and transported under temperatures
between -25 - 70 °C.
Operating
Designed for use at standard room temperatures (+5 to +40°C).
Air Pressure: 70 - 106 kPa
Relative Humidity: 15 - 93 %
After storage or transport at other temperatures leave it in a
room with a suitable temperature until it reaches a safe operating
temperature.
Medical electrical equipment requires special
precautions regarding electromagnetic
compatibility (EMC). Portable or mobile radio
communication equipment may affect the
medical electrical equipment, and should
be kept minimum 25 cm (10 inches) from the
hoists electronics.

Molift Air Tilt / www.etac.com
4
Installation
The hoist is marked with Safe Working Load (SWL), this should
not exceed rail systems max load capacity.
Minimum required height from floor to rail is 210cm.
Mounting Molift Air Tilt Sling bar
ENSVNODAFIDENLFRITES
50cm
Min.
210cm
60cm
Hand control
The Molift Air Tilt Hand control has 2 buttons for lifting and
lowering and 2 buttons for tilting the sling bar. The hand control
has an indicator light that will illuminate when battery level is low
and the hoist requires charging
Do not pull hand control to move the hoist
along the rail.
1. Place sling bar under hoist and lower lifting band. DO NOT
LIFT SLING BAR UP TO LIFTING BAND.
2. Align the sling bar in connection point.
3. Push button on locking pin and insert all the way through.
4. Make sure locking pin is properly fastened
5. Push and hold down the button on the locking pin, and pull
to remove locking pin.
6. Insert the plug on cable from actuator on Molift Air Tilt
Slingbar into the connector on Molift Air 350 Tilt.
7. Tighten the connectors sealing ring by turning it clockwise.

Molift Air Tilt / www.etac.com
5
Transfer
Plan the lifting operation in advance to ensure that it is as safe
and smooth as possible. Remember to work ergonomically.
Assess the risks and take notes. The caregiver is responsible for
the safety of the client. When moving the client, stand to the side
of the client you are lifting. Make sure that arms and legs do not
obstruct the seat, bed, etc. Keep eye contact with the client to
help them feel safe.
Using slings
Read User Manual for the sling prior to use.
Do not to use damaged or badly worn slings.
Lifting and lowering
Check that the sling is correctly fitted around the client. Stretch
the sling straps without lifting the client. Ensure that all four clips
of the sling are securely fastened to avoid the client slipping or
falling. Lift client and perform transfer.
General safety precautions
Only use clip slings that are adjusted to fit the client, type of
disability, size, weight and type of transfer.
If maximum load (SWL) differs between hoist
sling bar and body support unit, then the lowest
maximum load shall always be used.
Molift hoists shall only be used to lift clients.
Never use the hoist to lift or move objects of any
kind.
Make sure that all 4 sling clips are correctly fitted
to the clip studs on sling bar. Pull lifting band to
lock clips to stud. Pull upper band to unlock.
Do not push or pull client or the sling!
-CLICK-
-CLICK-

Molift Air Tilt / www.etac.com
6
Slings made by other manufacturers
Etac shall not be liable for faults or accidents that can occur when
using slings made by other manufacturers.
ENSVNODAFIDENLFRITES
Slings
This list of SilvaLea Clip Sling sling series are tested to be used
with Molift Air Tilt Sling bar.
The SilvaLea slings are available in sizes S – XL, in polyester.
Complete list of slings and sling bars can be found on etac.com.
Silvalea High Easy Toileting Sling Poly. (Toilet)
Size: Art.no:
S DA-ST3ASM
M DA-ST3AMED
L DA-ST3ALRG
XL DA-ST3AXLRG
Silvalea High Easy Sling Poly (HighBack)
Size: Art.no:
S HYASMCP
M HYAMEDCP
L HYALRGCP
XL HYAXLRGCP
Cleaning / Disinfection
Clean on a regular basis. Clean surfaces with a damp cloth
using an appropriate pH-neutral detergent. Do not use solvents
or strong liquids, this may damage surfaces on the hoist. For
disinfection when needed; use isopropyl alcohol. Avoid abrasive
cleaning products. Do not expose electronics to running water.
Make sure not to damage or remove labels
when cleaning.
Periodic inspection scope
If there is any problems that could jeopardize
someones safety, the accessory shall imme-
diately be taken out of service and marked
“out of order”. Do not use untill it is repaired.
This accessory shall be inspected as a part of the periodic inspec-
tion of the hoist it is used with.
Periodic Inspection shall be performed at least once a year or
more frequently if required by local requirements. The inspection
must be performed by service personnel authorized by Etac.
Contact Etac at molift@etac.com for training and
authorization or recommendation of an approved service partner.
Service scope
Service involves replacing the actuator and inspection/replace-
ment of worn parts. This must be carried out by authorized
personnel. Contact your local Etac dealer, or see etac.com for a
recommendation of an approved service partner.
Symptom Possible Cause/Action
The hoist does not respond to
hand control action.
Emergency Stop on the hoist
is activated. Deactivate by
pushing button back in.
The hoists electronics is
overheated.
Wait for it to cool down.
Hand control is not plugged in
properly. Hand control or plug
or cord can be broken and
should be replaced.
Actuator cable is not plugged
in properly.
Troubleshooting
Before use / Daily check
Inspection to be performed by caregiver daily or before use:
Make sure sling bar connection is properly connected and
secured.
Make sure wiring, hand control, clip studs or joints do not
have any visible damage.
If there are any faults or defects, the Molift Air Tilt needs to be
taken out of operation and marked ”out of order”.

Molift Air Tilt / www.etac.com
7
CE-försäkran
Produkten och de tillbehör som
beskrivs i denna bruksanvisning
överensstämmer med förordning (EU)
2017/745 av den 5 april 2017 som
medicinteknisk produkt, riskklass I.
Produkten har testats och godkänts
av tredje part i enlighet med stan-
darderna IEC 60601-1, IEC 60601-1-2
och EN/ISO 10535:2006.
Alla allvarliga incidenter som inträffar
och som är relaterade till produkten
ska rapporteras till tillverkaren och
den behöriga myndigheten i det
medlemsland där användaren och/
eller patienten bor.
Viktigt
Denna bruksanvisning innehåller viktiga säkerhetsinstruktioner
och information om användning av selen och tillbehören.
I denna bruksanvisning är användaren den som lyfts.
Vårdgivaren är den som använder lyften.
Den här symbolen indikerar viktig information
gällande säkerhet. Följ dessa anvisningar noga.
Läs igenom bruksanvisningen före användning!
Det är viktigt att förstå innehållet i
bruksanvisningen före försök att använda
utrustningen.
Besök www.etac.com för nedladdning av dokumentation för att
säkerställa att du har den senaste versionen.
Allmänt
Användningsvillkor
Lyft och förflyttning av en användare utgör alltid en viss risk och
endast utbildad personal får lov att använda den utrustning och
de tillbehör som omfattas av denna bruksanvisning. Skensystemet
måste monteras av auktoriserad personal med tillämpliga
monteringsanvisningar.
Ändringar och användning av komponenter som tillverkats av andra
tillverkare.
Vi rekommenderar endast användning av komponenter och
reservdelar från Molift. Försäkran om överensstämmelse är inte
giltig och Etac ansvarar inte för garantin om några ändringar har
utförts på produkten. Etac ansvarar inte för fel eller olyckor som
kan uppstå vid användning av komponenter som tillverkats av
andra tillverkare.
Lyften är inte avsedd att användas av användaren som lyfts. Om
en lyft ska användas av en användare som bor ensam ska någon
typ av kommunikationsutrustning installeras i lyftens användnings-
område så att användaren kan tillkalla hjälp i händelse av en nöd-
situation. Detta kan till exempel vara montering av ett larmsystem
eller tillgång till en lämpligt placerad telefon mm.
Endast auktoriserad personal får öppna
lyften eller tillbehören för att utföra service
eller reparation. Det finns risk för skada från
roterande delar och elstöt.
Garantimeddelande
Två års garanti mot defekter i utförande och
material i våra produkter.
Ett års garanti på batterier.
Se www.etac.com för villkor.

Molift Air Tilt / www.etac.com
8
Molift Air Tilt
1
2
3
5
4
Komponenter:
1. Molift Air 350 Tilt
2. Snabbfrigöringssprint
3. Handenhet
4. Kabel för anslutning till Molift Air 350 Tilt
5. Molift Air Tilt Sling bar
Om Molift Air Tilt
Molift Air Tilt Sling bar är ett tillbehör till Molift Air 350 Tilt taklyft
som ett alternativ till standardbyglar. Molift Air Tilt består av en
sele med klämfästen (tillbehör) anslutna till en justerbar bygel
som stöds av ett elektriskt manöverdon som gör det möjligt för
vårdgivaren att styra och justera patientens läge, t.ex. från upprätt
till tillbakalutat läge.
Produktidentifiering
Produktetikett
1. Produktetikett, 2. Produktetikett, 3. Manöverdon (Linak),
4.Etikett med serienummer
Varningsetiketter och symboler
Detaljerad förklaring av symboler som används:
NODAFIDENLFRITES ENSV
Molift Air 350 Tilt
Gross weight 361kg / 797lbs
SWL 350kg / 772lbs
24V SLA, IP24
Duty cycle 10%
Molift Air Tilt Sling bar
Gross weight 279,5kg / 616lbs
SWL 272kg / 600lbs
24V SLA, IP24
Duty cycle 10%
Uppfyller alla relevanta
EG-direktiv
IEC 60417-5172:
utrustning i klass II
Se bruksanvisningen Bruksanvisning
Endast för inomhusbruk
Manufacturer
Högsta användarvikt
YYYY-MM-DD
Date of manufacture
Får inte kasseras i hushålls-
avfall
WEEE-direktivet 2002/96/
EG
Catalogue number
Indikator för låg batterinivå
(handenhet)
Serial number
Kontrollmärke om förore-
ning i Kina (anger även
återvinningsbarhet) enligt
RoHS-lagstiftning i Kina
Medical Device
Märkning om regle-
ringsöverenstämmelse
iAustralien –
EMC, elsäkerhet (RCM)

Molift Air Tilt / www.etac.com
9
Förhållande mellan arbete och paus/arbetscykel.
Arbetscykel 10 %. Intervall enligt standard ISO-SS 10535)
Tekniska data
Säker arbetslast (SWL)
272 kg
Enhetsvikt (förutom handenhet och kablar):
7,3 kg
Manöverdonshastighet
12,6 - 10,4 mm/sekund
Skyddsklass:
Handenhet IP24
Manöverdon IPX4
Driftkraftsknapp
Knappar på handenheten: 3,4 N
Material:
stål, plast
Förväntad livstid:
Förväntad livstid på 30 000 cykler SWL eller 10 år.
Mått:
L x B x H (Längd, bredd, höjd)
66 x 58 x 50 - 79 cm
Lutningsintervall:
75 grader
75°
47cm
50-79cm
58cm
58cm
Elektronik
Transport och förvaring
Lyften kan förvaras och transporteras vid temperaturer mellan
-25 - 70 °C.
Drift
Konstruerad för användning vid standardmässiga rums-
temperaturer (+5 till +40 °C).
Lufttryck: 70 - 106 kPa
Relativ luftfuktighet: 15 - 93 %
Efter förvaring eller transport vid andra temperaturer ska den
förvaras i ett rum med lämplig temperatur tills den uppnår en
säker drifttemperatur.
Medicinsk elektrisk utrustning kräver
särskilda försiktighetsåtgärder avseende
elektro magnetisk kompatibilitet (EMC).
Flyttbar eller bärbar radiokommunikations-
utrustning kan påverka den medicinska
elektriska utrustningen och ska placeras
minst 25 cm från lyftens elektronik.

Molift Air Tilt / www.etac.com
10
Installation
Lyften markeras med säker arbetslast (SWL). Denna får inte
överstiga skensystemets högsta lastkapacitet.
Minsta erfordrade höjd från golv till skena är 210 cm.
Montering av Molift Air Tilt Sling bar
50cm
Min.
210cm
60cm
Handenhet
Molift Air Tilt Hand control har två knappar för lyftning och sänk-
ning samt två knappar för lutning av bygeln. Handenheten har
en indikatorlampa som tänds när batterinivån är låg och lyften
behöver laddas.
Tryck inte på handenheten för att flytta
lyften utmed skenan
1. Placera bygeln under lyften och sänk lyftremmen. LYFT INTE
BYGELN UPP TILL LYFTREMMEN.
2. Rikta in bygeln i anslutningspunkten.
3. Tryck på låssprinten och för in den hela vägen genom hålet.
4. Säkerställ att låssprinten är ordentligt fäst.
5. Tryck och håll ner knappen på låssprinten och dra för att ta
bort låssprinten.
6. För in kabelns plugg från manöverdonet på Molift Air Tilt
Sling bar in ianslutningsdonet på Molift Air Tilt.
7. Dra åt anslutningsdonens förslutningsring genom att vrida
medurs.
NODAFIDENLFRITES ENSV

Molift Air Tilt / www.etac.com
11
Förflyttning
Planera lyftningen i förhand för att säkerställa att det går så
säkert och smidigt som möjligt. Kom ihåg att arbeta ergonomiskt.
Bedöm riskerna och anteckna. Vårdgivaren ansvarar för använ-
darens säkerhet. Stå vid sidan om användaren som du lyfter när
du förflyttar användaren. Se till att armar och ben inte hindrar
stolen, sängen osv. Håll ögonkontakt med användaren för att
hjälpa användaren att känna sig säker.
Användning av selar
Läs igenom bruksanvisningen för selen före
användning.
Använd inte skadade eller utslitna selar.
Lyftning och sänkning
Kontrollera att selen är korrekt fäst runt användaren. Sträck
selremmarna utan att lyfta användaren. Se till att att selens alla
fyra klämmor är ordentligt fästa för att undvika att användaren
halkar eller faller.Lyft användaren och utför förflyttningen.
Allmänna säkerhetsåtgärder
Använd endast selar med klämmor som justeras för att anpassas
till användaren, typ av funktionsnedsättning, storlek, vikt och typ
av överföring.
Om den högsta arbetslasten (SWL) skiljer sig mellan
lyftens selbygel och enheten för kroppsstöd ska
alltid den lägsta maximala arbetsbelastningen
användas.
Molift lyftar ska endast användas till att lyfta
användare. Använd aldrig lyften för att förflytta
någon typ av föremål.
Säkerställ att alla fyra selklämmorna är ordentligt
fästa på bygelns klämstift. Dra i lyftremmen för att
låsa klämmorna på stiften. Dra i den övre remmen
för att låsa upp.
Tryck inte på eller dra inte i användaren eller selen!
-CLICK-
-CLICK-

Molift Air Tilt / www.etac.com
12
Selar som tillverkats av andra tillverkare.
Etac ansvarar inte för fel eller olyckor som kan uppstå vid använd-
ning av selar som tillverkats av andra tillverkare.
Selar
Listan över selserien SilvaLea sele med klämmor är testade för att
användas tillsammans med Molift Air Tilt Sling bar.
SilvaLea selar finns tillgängliga i storlekarna S - XL i polyester.
Fullständig lista över selar och selbyglar finns på etac.com.
Silvalea High Easy Toileting Sling Poly. (Toalett)
Storlek: art.nr:
S DA-ST3ASML
M DA-ST3AMED
L DA-ST3ALRG
XL DA-ST3AXLRG
Silvalea High Easy Sling Poly (hög rygg)
Storlek: art.nr:
S HYASMCP
M HYAMEDCP
L HYALRGCP
XL HYAXLRGCP
Rengöring/desificering
Rengör regelbundet. Rengör ytorna med en fuktig trasa och
lämpligt pH-neutralt rengöringsmedel. Använd inte lösningsmedel
eller starka vätskor. Det kan skada lyftens ytor. Använd isopropyl-
alkohol när desinficering behövs. Undvik slipande rengörings-
medel. Utsätt inte elektronik för rinnande vatten.
Se till att inte skada eller avlägsna etiketter
vid rengöring.
Periodiskt inspektionsintervall
Vid eventuella problem som kan äventyra
någons säkerhet ska tillbehöret omedelbart
tas ur bruk och markeras ”ur funktion”.
Använd inte förrän den är reparerad.
Detta tillbehör ska inpekteras som en del i den periodiska inspek-
tionen av lyften som det används tillsammans med.
Periodisk inspektion ska utföras minst en gång per år eller oftare
om det krävs i lokala föreskrifter. Inspektionen måste utföras av
servicepersonal som är auktoriserad av Etac. Kontakta Etac på
molift@etac.com för utbildning och auktorisering eller rekom-
mendation om en godkänd servicepartner.
Serviceintervall
Service omfattar utbyte av manöverdon och inspektion/utbyte
av utslitna delar. Den måste utföras av auktoriserad personal.
Kontakta din lokala återförsäljare för Etac eller besök etac.com
för rekommendation om en godkänd servicepartner.
Symtom Möjlig orsak/åtgärd
Lyften svarar inte på åtgärd
med handenheten
Akutstopp på lyften är
aktiverad. Inaktivera genom
att trycka in knappen igen.
Lyftens elektronik är
överhettad.
Vänta tills den avsvalnat.
Handenheten har inte kopp-
lats in ordentligt. Handenhet,
stickpropp eller sladd kan vara
trasig och ska bytas ut.
Manöverdonets kabel är inte
ordentligt inkopplad.
Felsökning
Före användning/daglig kontroll
Inspektion som ska utföras av vårdgivaren dagligen eller före
användning:
Säkerställ att selbygelns anslutning är ordentligt ansluten och
säkrad.
Säkerställ att sladdar, handenhet, klämstift eller skarvar inte
har några synliga skador.
Vid eventuella fel eller defekter ska Molift Air Tilt tas ur bruk och
markeras ”ur funktion”.
NODAFIDENLFRITES ENSV

Molift Air Tilt / www.etac.com
13
Samsvarserklæring
Produktet og tilhørende tilbehør, som
er beskrevet i denne bruksanvisnin-
gen, er i samsvar med forordning
(EU) 2017/745 av 5. april 2017 - som
medisinsk utstyr, risikoklasse I.
Produktet er testet og godkjent av en
tredjepart i henhold til standardene
IEC 60601-1, IEC 60601-1-2 og EN/ISO
10535:2006.
Alle alvorlige hendelser som oppstår
i forbindelse med enheten, skal rap-
porteres til produsenten og kompe-
tent myndighet i landet der brukeren
og/eller pasienten befinner seg.
Viktig
Denne bruksanvisningen inneholder viktige sikkerhetsinstruksjoner
og informasjon om bruk av seilet og tilbehøret.
Brukeren i denne bruksanvisningen er personen som løftes.
Pleieren er personen som betjener løfteren.
Dette symbolet indikerer viktig informasjon
om sikkerhet. Disse instruksjonene må følges
nøye.
Les bruksanvisningen før bruk!
Det er viktig å forstå bruksanvisningens
innhold fullt og helt før utstyret betjenes.
Gå til www.etac.com for å laste ned dokumentasjon, slik at du
har den nyeste utgaven.
Generelt
Bruksbetingelser
Løfting og forflytning av en bruker medfører alltid en viss risiko,
og utstyret og tilbehøret som er beskrevet i denne bruksanvis-
ningen, skal bare brukes av kvalifisert personale. Skinnesystemet
skal installeres av sertifisert personell i samsvar med gjeldende
installasjonsanvisning.
Endringer og bruk av komponenter som er laget av andre produsenter.
Vi anbefaler at det kun brukes Molift komponenter og reserve-
deler. Samsvarserklæringen blir ugyldig, og Etac står ikke ansvar-
lig for garantien dersom utstyret er utsatt for modifikasjoner. Etac
har intet ansvar for eventuelle feil eller ulykker som kan oppstå
ved bruk av komponenter fra andre produsenter.
Løfteren er ikke ment for å betjenes av brukeren som løftes. Hvis
en løfter skal brukes av en bruker på egenhånd, må det være
installert kommunikasjonsutstyr i området der løfteren brukes,
slik at brukeren kan tilkalle hjelp i en nødssituasjon. Det kan for
eksempel være montering av et alarmsystem eller en telefon med
egnet plassering, o.l.
Bare autorisert personale har tillatelse til å
åpne løfteren eller tilbehøret for å utføre
service eller reparasjoner. Fare for skade som
følge av roterende deler og elektrisk støt.
Garantimelding
To (2) års produktgaranti mot feil i materialer
og arbeidsutførelse.
Ett (1) års batterigaranti.
Les vilkår og betingelser på www.etac.com.

Molift Air Tilt / www.etac.com
14
Molift Air Tilt
1
2
3
5
4
Komponenter:
1. Molift Air 350 Tilt
2. Hurtigutløserpinne
3. Håndkontroll
4. Kabel for tilkobling til Molift Air 350 Tilt
5. Molift Air Tilt Sling bar
Om Molift Air Tilt
Molift Air Tilt Sling bar er et tilbehør til Molift Air 350 Tilt takløfter
og er et alternativ til standard sprederstenger. Molift Air Tilt
har klipshekter for tilkobling av tilbehør, og med den justerbare
sprederstangen, som støttes av en elektrisk aktuator. Den gjør det
mulig for pleieren å kontrollere og tilpasse pasientens posisjon,
f.eks. fra sittende til liggende.
Produkt-ID
Produktetikett
1. Produktetikett, 2. Produktetikett, 3. Aktuatoretikett (Linak),
4.Etikett med serienummer
Advarselsetiketter og symboler
Symboler benyttet på produktet, forklart mer detaljert:
SVNODAFIDENLFRITES EN
Molift Air 350 Tilt
Gross weight 361kg / 797lbs
SWL 350kg / 772lbs
24V SLA, IP24
Duty cycle 10%
Molift Air Tilt Sling bar
Gross weight 279,5kg / 616lbs
SWL 272kg / 600lbs
24V SLA, IP24
Duty cycle 10%
Samsvar med alle relevante
EF-direktiver
IEC 60417-5172:
Utstyr i klasse II
Se bruksanvisningen Driftsinstruksjoner
Kun innendørs bruk
Manufacturer
Maksimal brukervekt
YYYY-MM-DD
Date of manufacture
Må ikke kastes med hushold-
ningsavfall.
WEEE Direktiv
2002/96/EF
Catalogue number
Indikator for lavt batteri
(håndkontroll)
Serial number
Kontrollmerkning for foru-
rensning fra Kina (indikerer
også gjenvinningsgrad)
China RoHS-lovgivning
Medical Device
Regulatorisk samsvarsmerkn-
ing for Australia –
EMC, electrisk sikkerhet
(RCM)

Molift Air Tilt / www.etac.com
15
Driftspauser/driftssyklus.
Driftssyklus 10 %. (Intervaller i henhold til standarden ISO-EN
10535)
Tekniske data
Sikker arbeidsbelastning (SWL)
272 kg (600 lbs)
Enhetens vekt (ekskl. håndkontroll og kabler):
7,3 kg (16 lbs)
Aktuatorhastighet
12,6–10,4 mm/sekund (0,49–0,40 tommer/sekund)
Beskyttelsesklasse:
Håndkontroll IP24
Aktuator IPX4
Betjeningskraft, knapp
Knapper på håndsettet: 3,4 N
Materiale:
Stål, plast
Forventet levetid:
Forventet levetid på 30 000 sykluser med sikker arbeids-
belastning eller 10 år.
Dimensjoner:
L x B x H (Lengde, Bredde)
66 x 58 x 50–79 cm
(26 x 22,8 x 19,7–33,5 tommer)
Vinklingsområde:
75 grader
75°
47cm
50-79cm
58cm
58cm
Elektronikk
Transport og lagring
Løfteren kan lagres og transporteres ved temperaturer mellom
-25 til +70 °C.
Drift
Konstruert for bruk ved vanlig romtemperatur (+5 til +40 °C).
Lufttrykk: 70 – 106 kPa
Relativ fuktighet: 15 – 93 %
Etter lagring eller transport i andre temperaturer må løfteren stå i
et rom med egnet temperatur til den når sikker driftstemperatur
Medisinsk elektrisk utstyr krever spesielle for-
holdsregler med hensyn til elektromagnetisk
kompatibilitet (EMK). Flyttbart eller bærbart
radiokommunikasjonsutstyr kan påvirke det
medisinske elektriske utstyret, og skal holdes
minst 25 cm (10 tommer) fra elektronikken til
løfteren.

Molift Air Tilt / www.etac.com
16
Installasjon
Løfteren er merket med sikker arbeidsbelastning (SWL), som ikke
skal overskride skinnesystemets maksimale lastekapasitet.
Minimum høyde fra gulv til skinner er 210 cm.
Montere Molift Air Tilt Sling bar
50cm
Min.
210cm
60cm
Håndkontroll
Håndkontrollen har 2 knapper for løfting og senking, og 2 knapper
for vinkling av løftebøylen. Håndkontrollen har en indikatorlampe
som tennes når batterinivået er lavt og løfteren må lades.
Ikke trekk i håndkontrollen for å flytte
løfteren langs skinnen
1. Plasser sprederstangen under løfteren og senk løftebåndet.
IKKE LØFT SPREDERSTANGEN OPP TIL LØFTEBÅNDET.
2. Juster løftebøylen i koblingspunktet.
3. Trykk på knappen på låsepinnen og sett den helt gjennom.
4. Kontroller at låsepinnen sitter som den skal.
5. Trykk og hold inne knappen på låsepinnen, og trekk ut
låsepinnen for å fjerne den.
6. Sett pluggen på kabelen fra aktuatoren på Molift Air Tilt
Sling bar inn i tilkoblingen på Molift Air 350 Tilt.
7. Stram forseglingsringene på tilkoblingene ved å skru dem
med klokken.
SVNODAFIDENLFRITES EN

Molift Air Tilt / www.etac.com
17
Forflytning
Planlegg løfteoperasjonen på forhånd for å sikre at den foregår
mest mulig trygt og uproblematisk. Husk å arbeide ergonomisk.
Vurder risikoen og ta notater. Pleieren er ansvarlig for brukerens
sikkerhet. Stå ved siden av brukeren som løftes under forflytning
av brukeren. Kontroller at armer og ben ikke kommer i veien
for sete, seng osv. Hold øyekontakt med brukeren slik at ved-
kommende føler seg trygg.
Bruke seil
Les bruksanvisningen for seilet før bruk.
Ikke bruk skadede eller veldig utslitte seil.
Løfting og senking
Kontroller at seilet sitter som det skal rundt brukeren. Stram opp
seilstroppene uten å løfte brukeren. Kontroller at alle de fire
løkkene på seilet er riktig festet, for å unngå at brukeren glir eller
faller. Løft pasienten og utfør forflytningen.
Generelle sikkerhetsregler
Bruk bare seilløkker som er justert slik at de passer til brukeren,
type funksjonsnedsettelse, størrelse, vekt og type forflytning.
Hvis de maksimale sikre arbeidsbelastningene for
løftebøylen og kroppsstøtten er forskjellige, skal
den laveste maksimale lasten alltid brukes
Molift-løftere skal bare brukes til å løfte brukere.
Bruk aldri løfteren til å løfte eller flytte gjen-
stander av noe slag.
Sørg for at alle 4 seilklipsene er festet på riktig
måte til festehakene på sprederstangen. Dra i
løftebåndet for å låse klipsene fast i festehaken.
Dra i det øvre båndet for å låse opp.
Ikke dytt eller dra i brukeren eller seilet!
-CLICK-
-CLICK-
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
-
 45
45
-
 46
46
-
 47
47
-
 48
48
-
 49
49
-
 50
50
-
 51
51
-
 52
52
-
 53
53
-
 54
54
-
 55
55
-
 56
56
-
 57
57
-
 58
58
-
 59
59
-
 60
60
-
 61
61
-
 62
62
-
 63
63
-
 64
64
in altre lingue
- English: Molift Air Tilt User manual
- français: Molift Air Tilt Manuel utilisateur
- español: Molift Air Tilt Manual de usuario
- Deutsch: Molift Air Tilt Benutzerhandbuch
- Nederlands: Molift Air Tilt Handleiding
- dansk: Molift Air Tilt Brugermanual
- svenska: Molift Air Tilt Användarmanual
- suomi: Molift Air Tilt Ohjekirja
Documenti correlati
-
 Molift Air 500 Manuale utente
Molift Air 500 Manuale utente
-
 Molift Air 350 Manuale utente
Molift Air 350 Manuale utente
-
 Molift Air 500 Manuale utente
Molift Air 500 Manuale utente
-
 Molift Mover 180 Manuale utente
Molift Mover 180 Manuale utente
-
 Molift Quick Raiser 205 Manuale utente
Molift Quick Raiser 205 Manuale utente
-
 Molift Mover 300 Manuale utente
Molift Mover 300 Manuale utente
-
Molift Smart 150 Manuale utente
-
 Molift RgoSling MediumBack Plus Manuale utente
Molift RgoSling MediumBack Plus Manuale utente
-
 Molift RgoSling Active Manuale utente
Molift RgoSling Active Manuale utente
-
 Molift Rail System Manuale utente
Molift Rail System Manuale utente
Altri documenti
-
Invacare 2478444 Manuale utente
-
Gima 43455 Manuale del proprietario
-
Invacare Robin Mover Manuale utente
-
Ferm LHM1008 Manuale del proprietario
-
Ingersoll-Rand VL2-015 Istruzioni per l'uso
-
Medisana PT 100 Manuale del proprietario
-
Gima 43460 Manuale del proprietario
-
Vermeiren Albatros II Manuale utente
-
Tecnosystemi Electric lifter Manuale del proprietario
-
Burmeier DALI Istruzioni per l'uso








































































