
EN
Regulation (EU)
2017/745
X-TRIM 4
855.12

2
Original version in Italian dated 10/01/2022
Download the digital version - Scarica la versione digitale

855.12 X-TRIM 4
www.kong.it
3
Original version in Italian dated 10/01/2022
TABLE OF CONTENTS
1 - SYMBOLS AND SUPPORT 4
1.1 Symbols 4
1.2 Support 4
2 - GENERAL INFORMATION 5
3 - TECHNICAL FEATURES 6
3.1 Parts terminology and materials 6
3.2 Dimensions 7
3.3 Capacity 7
3.4 Optional components and spare parts 7
4 - SPECIFIC INFORMATION 8
4.1 Applications 8
4.2 Assembly 8
4.3 Mounting the “HRP” Head immobiliser 10
4.4 Immobilising the patient 11
4.5 Unlocking the patient 14
5 - MAINTENANCE AND REPAIR 15
5.1 General Information 15
5.2 Maintenance 15
5.3 Repair 15
6 - STORAGE 16
7 - CHECKS, INSPECTIONS AND REVISIONS 17
7.1 Checks 17
7.2 Inspections 17
7.3 Revisions 17
8 - DURATION AND WARRANTY 18
8.1 Duration 18
8.2 Disposal 18
8.3 Warranty 18
8.4 Legal requirements 18
9 - LABELLING AND SYMBOLS 19
9.1 Labelling 19
9.2 Symbols 19
10 - DOCUMENTS 20
10.1 Inspections and revisions register 20
10.2 Maintenance and repair register 21
10.3 Declaration of conformity UE 22
11 - LIST OF APPLIED AND REFERENCE STANDARDS 23
11.1 Applied standards 23
11.2 Standards used as reference 24

4
Original version in Italian dated 10/01/2022
CHAPTER
To make reading this manual comfortable and clear, the following are the symbols used to
manage important warnings, for correct and safe use of the device.
1.1
SYMBOLS
REQUISITE FOR CORRECT USE
It identifies the presence of information required to use the device correctly.
INFORMATIVE REQUISITE
It identifies the presence of useful and general information, to guide the
user to consciously use the device and/or carry out the actions.
It identifies that the Medical Device is manufactured, designed and pr-
duced in compliance with the provisions of the General Safety and Perfor-
mance Requirements of Regulation (EU) 2017/745 (Class I medical device,
in compliance with classification rule 1 as indicated in the attachment VIII).
1
SYMBOLS
AND SUPPORT
For information, contact Kong Customer Service by:
- telephone +39 0341 630506,
- fax +39 0341 641550,
- email: [email protected],
or write to KONG S.p.A. – Via XXV Aprile, 4 – 23804 Monte Marenzo LC - ITALY.
To facilitate assistance operations, always communicate or indicate the serial number (SN)
indicated on the label applied to the Medical Device.
1.2
SUPPORT

855.12 X-TRIM 4
www.kong.it
5
Original version in Italian dated 10/01/2022
Warning: not suitable for
use in an ATEX environ-
ment (Directive 94/9/CE)
The information provided by the manufacturer
(hereinafter referred to as information)
must be read and clearly understood by
the user prior to using the Medical Device
(MD hereafter). The information regards the
description of the features, performance,
assembly, disassembly, maintenance,
preservation, disinfection, etc. of the device.
Even though the information offers tips on
use, this information shall not be considered
as a user manual under actual conditions of
use.
WARNINGS AND
LIMITATIONS OF USE:
- this device shall be strictly used by people
who are physically fit, trained (instructed
and taught) to use the device and with
specific experience regarding moving
the patient or, during training activities,
by people under direct supervision of the
trainers/ supervisors who guarantee the
safety thereof,
- do not use the device before fully reading
and understanding this user manual,
- strictly follow the manufacturer’s
information, improper use of the device is
hazardous,
- modifying and/or repairing the device is
strictly forbidden,
- all checks described in chapter 7 must
be carried out prior to and after using
the device. In case of any doubt on
the efficiency of the device, the user
must replace it immediately,
- non-compliant use, deformations, falls, wear,
chemical contamination, exposing textile/
plastic components/devices to temperature
below -30°C or above +50°C and metal
components/devices to temperatures
2
GENERAL INFORMATION
exceeding 100°C are some examples of
causes that can reduce, limit and end the
life of the device,
- Prior to any rescue operation, be keen not to
exceed the capacity indicated in paragraph
3.3,
- in order to reduce risks of exposure to /
transmission of infectious diseases, clean
and disinfect the device as indicated in
chapter 5,
- improper use of the patient immobilisation
systems can jeopardise the safety of the
patient,
- always check the compatibility of the devices
used alongside the device by consulting the
relative manufacturer’s information,
- use of spare parts or optional components
different from the ones indicated in
paragraph 3.4 can be hazardous,
- do not expose the device to sources of heat
and at contact with chemical substances.
Reduce exposure to direct sunlight as
much as possible. At low temperatures and
in the presence of humidity, formation of ice
could reduce flexibility and increase cutting
and abrasion-related risks on textile and
synthetic devices.
- Report to the manufacturrer and the
competent authority of the member state
where the user and / or patient is established
any serious incident occurring in relation to
the MD.
All our devices are tested/ checked piece
by piece in compliance with the procedures
laid down by the Quality System certified
in accordance with the UNI EN ISO
9001 standard. Laboratory tests, testing,
information and standards do not always
reproduce the practical result. Thus, the
results obtained under the actual conditions
of use of the device in the natural environment
may differ, even considerably at times.
The best information lies in the continuous
practical use under the supervision of
skilled/expert/qualified people.
CHAPTER 2

6
Original version in Italian dated 10/01/2022
A – Carbon fibre spinal board
B – Carbon fibre spars
C – Nylon hinge
D – Nylon rivets
E – Brass rear hinges
F – Carbon fibre crossbars
G – Taylan Belt
TECHNICAL
FEATURES
3.1 PARTS TERMINOLOGY AND MATERIALS
Fig.1
CHAPTER 3
EE
EE
CD
AB
B
G
F F
F

855.12 X-TRIM 4
www.kong.it
7
Original version in Italian dated 10/01/2022
How to use Capacity
Manual handling 150 kg
Prior to any rescue operation, ensure not to exceed the specified capacity!
3.2 DIMENSIONS
Open: 182x42x4 cm
Folded: 91x24x8 cm
Weight: 6,8 kg
3.3 CAPACITY
The load capacity of the “X-TRIM 4” spinal board is defined according to the results of the
laboratory tests and the recommended safety coefficients.
3.4 OPTIONAL COMPONENTS AND SPARE PARTS
3.4.1 Optional components
- 855.06 HRP: head immobilisation device
C - Nylon hinge.
D - Rivets for fixing the nylon hinge.
G - Taylan Belt.
Rivet breakage is provided to prevent damage to the spinal board should it be
bent leaving material inside.
3.4.2 Spare parts
The methods of connection and use of the optional components are defined in the relative
user instructions.

8
Original version in Italian dated 10/01/2022
SPECIFIC
INFORMATION
4.1 APPLICATION
4.2 ASSEMBLY
The “X-TRIM 4” spinal board is a medical device for patient immobilisation suitable for any
type of rescue, including rough terrain. The minimal weight and thickness facilitates roll-
on manoeuvres. Decisions concerning moving and immobilising the patient, as well as the
duration, method to be used and combination with other devices, shall be taken and executed
by expert and trained personnel only.
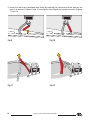
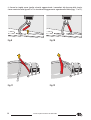
a) Open the spinal board (Fig. 2),
b)rotate the crossbars (F) until they lock into their slots (fig. 3),
Fig.2
Fig.3
CHAPTER 4

855.12 X-TRIM 4
www.kong.it
9
Original version in Italian dated 10/01/2022
Fig.4
c) Slide the stiffening spars until they lock into the lateral grooves of the spinal board (fig. 4),

10
Original version in Italian dated 10/01/2022
4.3 MOUNTING THE HRP HEAD IMMOBILISER
a) Insert the Velcro straps into the slots of the head immobiliser and spinal board (Fig. 5).
Fig.5
b) Attach the head immobiliser by pulling and overlapping the Velcro straps on the underside
of the spinal board (fig. 6).
Fig.6

855.12 X-TRIM 4
www.kong.it
11
Original version in Italian dated 10/01/2022
a) place the patient’s head in the head immobiliser and immobilise it using the padded
straps (Figs. 7 and 8),
The head immobiliser is also suitable for immobilising patients with cervical
collars.
Fig.7
Fig.8
4.4 IMMOBILISING THE PATIENT
With the spinal board positioned on a flat and stable surface:
b) Position the “TAYLAN” belt as shown in figure 8,

12
Original version in Italian dated 10/01/2022
c) fasten the red straps (shoulders and torso) by hooking the connectors to the slots on the
board, as shown in Figures 9 and 10, and tighten them slightly by using the buckles (Figures
11 and 12),
Fig.9
Fig.11
Fig.10
Fig.12

855.12 X-TRIM 4
www.kong.it
13
Original version in Italian dated 10/01/2022
d) fasten the black straps (pelvis and legs) by hooking the connectors to the slots on the board
and slightly tighten them using the buckles (figs. 13 and 14)
e) conclude the immobilisation by using the longitudinal lock (fig. 15),
Fig.13
Fig.15
Fig.14
f) the patient fully immobilised on the spinal board can be moved by the operators.
Avoid prolonged contact with the patient’s skin with the device’s materials.

14
Original version in Italian dated 10/01/2022
Fig.18 Fig.19
a) Loosen the straps by using the buckles as shown in figure 16.
If the straps are removed from their buckles, they can be reinserted as shown
in figures 17-18-19,
Fig.16 Fig.17
4.5 UNLOCKING THE PATIENT

855.12 X-TRIM 4
www.kong.it
15
Original version in Italian dated 10/01/2022
The maintenance operations that must be carried out by the user are, in order:
a) cleaning: after every use, wash with warm (max. 40 C) potable water, possibly adding a
neutral detergent (e.g. Marseille soap). Rinse and leave to dry in a shaded place, away
from direct sources of heat,
b) disinfection, when deemed necessary or after prolonged inactivity (more than 21 days),
preceded by cleaning: wipe with a clean cloth , soaked in a water solution of sodium
hypochlorite (bleach) with a 0,1% concentration (1000 ppm). When blood or other bodily
fluids are present, the recommended concentration of sodium hypochlorite is 0,5%(5000
ppm)
Repair shall be strictly carried out by the manufacturer.
The user is allowed to replace the parts mentioned in paragraph 3.4.2 only,
with new and original parts.
The MD spinal board is made of materials that are highly resistant to wear and to external
agents. Despite this, the use conditions require maintenance operation and even repair in
particular conditions.
MAINTENANCE
AND REPAIR
5.1 GENERAL
5.2 MAINTENANCE
5.3 REPAIR
CHAPTER 5
Maintenance and repair operation must be recorded in a special register an
example of which is indicated in chapter 10.

16
Original version in Italian dated 10/01/2022
After cleaning, any disinfection and drying operations, keep the product and its optional
components in a place that is dry (relative humidity 40-90%), cool (temperature 5-40°
C) and safe (avoid UV radiations), chemically neutral (absolutely avoid salty and/or acid
environments), away from sharp edges, sources of heat, humidity, corrosive substances or
other possible jeopardising conditions.
Do not store this device wet!
6 STORAGE
CHAPTER 6
STORAGE

855.12 X-TRIM 4
www.kong.it
17
Original version in Italian dated 10/01/2022
Before and after every use, check the device and ensure that:
- it is appropriate for the use it was intended for,
- it has not been subjected to mechanical deformations and it neither has cracks nor shows
signs of wear,
- the textile parts do not have cuts, burns, chemical products residues, excessive hair, wear,
in particular check areas at contact with the metal components,
- markings, including labels, are readable.
The device must be inspected at least once a year, starting from the date of first use, by
expert personnel trained and approved by KONG S.p.A. The date of the first use and the
outcome of the inspection must be recorded in the Inspections and Servicing Register,
regarding which an example is indicated in chapter 10.
The device must be serviced by KONG S.p.A., or by expert personnel trained and approved
by KONG S.p.A. if:
- any malfunctions are detected,
- the result of the pre and post use check or of the inspections is negative.
The outcome of the servicing must be recorded in the Inspections and Servicing Register,
regarding which an example is indicated in chapter 10.
The device subject of servicing has a one-year warranty from the date of servicing.
CHECKS
INSPECTIONS
AND SERVICING
7.1 CHECKS
7.2 INSPECTIONS
7.3 SERVICING
CHAPTER 7

18
Original version in Italian dated 10/01/2022
PRODUCT LIFE
AND GUARANTEE
Carefully read the “Warnings and limitations of use” paragraph in chapter 2.
The duration of this device depends on the outcome of the at least one-yearly inspection
(paragraph 7.2) and possible servicing.
8.1 PRODUCT LIFE
Follow the rules in force in the country of use and the waste disposal procedures of the
reference hospital facility for proper disposal.
8.2 DISPOSAL
The manufacturer guarantees the compliance of the device with the regulations in force
at the time of production. Warranty covering defects is limited to defects regarding raw
materials and manufacturing defects. The warranty does not cover normal wear, oxidation,
damage caused by non-compliant use and/or in competition, by improper maintenance,
transportation, preservation or storage. Warranty will be immediately deemed null and void
should the device be modified or tampered with. Validity corresponds to the legal validity
of the country where the device was sold, as from the date of sale, by the manufacturer.
No claims shall be raised against the manufacturer once the aforementioned period has
expired. Any repair or replacement request under warranty must be accompanied by a
proof of purchase. Should the defect be recognised, the manufacturer undertakes to repair
or, replace or refund the device at his discretion. The manufacturer shall not be held liable
beyond the invoice price of the device in any case whatsoever.
8.3 GUARANTEE
Important: any devices that have not passed the pre-use, post-use and
periodic inspection or servicing must be eliminated and made unusable.
CHAPTER 8
Professional and free time activities are often regulated by special national laws which can
impose limits and/or obligations concerning the use of these devices. The user has the
obligation to know and apply such laws which could provide for limits different from those
indicated in this information.
8.4 LAW OBLIGATIONS

855.12 X-TRIM 4
www.kong.it
19
Original version in Italian dated 10/01/2022
Any changes to the positioning
of the symbols does not change
the content thereof.
Any changes to the positioning
of the symbols does not change
the content thereof.
KONG S.p.A.
Via XXV Aprile, 4
I - 23804 MONTE MARENZO (LC) - ITALY
Tel. +39 0341 630506
ITALY
2022 / 01
855021000KK - TAYLAN
123456 1234
UDI
MD
ITALY
2022 / 01
855.12 - MOD. X-TRIM 4
(01)08023577010724(21) 1234561234
KONG S.p.A.
Via XXV Aprile, 4
I - 23804 MONTE MARENZO (LC) - ITALY
Tel. +39 0341 630506
123456 1234
KONG S.p.A.
Via XXV Aprile, 4
I - 23804 MONTE MARENZO (LC) - ITALY
Tel. +39 0341 630506
ITALY
2022 / 01
855021000KK - TAYLAN
123456 1234
UDI
MD
ITALY
2022 / 01
855.12 - MOD. X-TRIM 4
(01)08023577010724(21) 1234561234
KONG S.p.A.
Via XXV Aprile, 4
I - 23804 MONTE MARENZO (LC) - ITALY
Tel. +39 0341 630506
123456 1234
LABELLING
AND SYMBOLS
CHAPTER 9
9.1 MEDICAL DEVICE LABEL
9.2 SEPARABLE COMPONENTS LABEL
9.3 SYMBOLS
Manufacurer's identification
Product identification code
Unique serial number
Country, year and month of production.
Unique Device Identifier
Medical Device
Consult the user manual
Compliant with Regulation (EU) 2017/745
UDI
MD
ITALY
YYYY / MM
Manufacurer's identification
Product identification code
Unique serial number
Country, year and month of production.
Unique Device Identifier
Medical Device
Consult the user manual
Compliant with Regulation (EU) 2017/745
UDI
MD
ITALY
YYYY / MM
Identification and traceability
Information and safety
Data matrix UDI
Readable with the Apps:
iGepir - BarValid - Barcode Scanner

20
Original version in Italian dated 10/01/2022
DOCUMENTS
SPINAL BOARD - Class I MD
REF. 855.12 MOD. X-TRIM 4
UDI-DI 08023577010724 SN
Date of first use
CHAPTER
10
10.1 INSPECTIONS AND SERVICING REGISTER
INSPECTIONS AND SERVICING
DATE I/S Description Outcome Supervisor
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
-
 45
45
-
 46
46
-
 47
47
-
 48
48
-
 49
49
-
 50
50


























































