
EN
Regulation (EU) 2017/745
Laden Sie die Übersetzung in Ihrer Sprache
herunter - Download the translation in your langua-
ge - Bájate la traducción en tu idioma - Télécharger
la traduction dans vostre langue - Scarica la
traduzione nella tua lingua
ZZVXXXXX Rev. 0
LECCO 2.0
870.04
QR
EN

2
original version in Italian dated 10/01/2022
Download the digital version - Scarica la versione digitale

870.04 LECCO 2.0www.kong.it
3
original version in Italian dated 10/01/2022
TABLE OF CONTENTS
1 - SYMBOLS AND SUPPORT 4
1.1 Symbols 4
1.2 Support 4
2 - GENERAL INFORMATION 5
3 - TECHNICAL FEATURES 6
3.1 Terminology and materials of the parts 6
3.2 Technical data 8
3.3 Optional components and spare parts 9
4 - SPECIFIC INFORMATION 16
4.1 Intended use 16
4.2 Preparing the scoop stretcher 17
4.3 Positioning and immobilising the patient 20
4.4 Transporting the patient 22
4.5 Disassembling the scoop stretcher 25
4.6 Removing the sheet from the metal structure 26
4.7 Fixing the sheet into the metal structure 26
5 - MAINTENANCE AND REPAIR 30
5.1 General 30
5.2 Maintenance 30
5.3 Repair 30
6 - STORAGE 31
7 - CHECKS, INSPECTIONS AND SERVICING 32
7.1 Checks 32
7.2 Inspections 32
7.3 Servicing 32
8 - DURATION AND WARRANTY 33
8.1 Duration 33
8.2 Disposal 33
8.3 Warranty 33
8.4 Law obligations 33
9 - LABELLING AND SYMBOLS 34
9.1 Medical Device Label 34
9.2 Separable components label 34
9.2 Symbols 34
10 - DOCUMENTS 35
10.1 Recording inspections and servicing 35
10.2 Maintenance and repair register 36
10.3 EU declaration of conformity (facsimile) 37
11 - LIST OF APPLIED AND REFERENCE STANDARDS 38
11.1 Applied standards 38
11.2 Standards used as reference 39

4
original version in Italian dated 10/01/2022
CHAPTER
For the sake of comfort and clarity while reading this manual, below are the symbols used
for handling important warnings for a proper and safe use of the device.
1.1
SYMBOLS
REQUIREMENT FOR PROPER USE
It identifies the presence of information for proper use of the device.
INFORMATION REQUIREMENT
It identifies the presence of useful and general information which guides
the reader towards a conscious use of the device and/or performance of
actions.
It identifies that the Medical Device is manufactured, designed and pro-
duced in compliance with the provisions of the General Safety and Perfor-
mance Requirements of Regulation (EU) 2017/745 (Class I medical device,
in compliance with classification rule 1 as indicated in the attachment VIII).
1
SYMBOLS
AND SUPPORT
For information please contact Kong Customer Support Service by:
- telephone +39 0341 630506
- fax +39 0341 641550
- email: [email protected]
or write to KONG S.p.A. – Via XXV Aprile, 4 – 23804 Monte Marenzo LC - ITALY.
To facilitate support operations, please always communicate or state the serial number (SN)
indicated on the label fixed to the Medical Device.
1.2
SUPPORT

870.04 LECCO 2.0www.kong.it
5
original version in Italian dated 10/01/2022
Warning: not suitable for
use in an ATEX environment
(Directive 94/9/CE)
The information provided by the manufacturer
(hereinafter referred to as information)
must be read and clearly understood by
the user prior to using the Medical Device
(MD hereafter). The information regards the
description of the features, performance,
assembly, disassembly, maintenance,
preservation, disinfection, etc. of the device.
Even though the information offers tips on
use, this information shall not be considered
as a user manual under actual conditions of
use.
WARNINGS AND
LIMITATIONS OF USE:
- this device shall be strictly used by people
who are physically fit, trained (instructed
and taught) to use the device and with
specific experience regarding moving
the patient or, during training activities,
by people under direct supervision of the
trainers/ supervisors who guarantee the
safety thereof,
- do not use the device before fully reading
and understanding this user manual,
- strictly follow the manufacturer’s
information, improper use of the device is
hazardous,
- modifying and/or repairing the device is
strictly forbidden,
- all checks described in chapter 7
must be carried out prior to and
after using the device. In case of any
doubt on the efficiency of the device,
the user must replace it immediately,
- non-compliant use, deformations, falls,
wear, chemical contamination, exposing
textile/plastic components/devices
to temperature below -30°C or above
2
GENERAL INFORMATION
+50°C and metal components/devices to
temperatures exceeding 100°C are some
examples of causes that can reduce, limit
and end the life of the device,
- Prior to any rescue operation, be keen
not to exceed the capacity indicated in
paragraph 3.3,
- in order to reduce risks of exposure to /
transmission of infectious diseases, clean
and disinfect the device as indicated in
chapter 5,
- improper use of the patient immobilisation
systems can jeopardise the safety of the
patient,
- always check the compatibility of the
devices used alongside the device by
consulting the relative manufacturer’s
information,
- use of spare parts or optional components
different from the ones indicated in
paragraph 3.4 can be hazardous,
- do not expose the device to sources of heat
and at contact with chemical substances.
Reduce exposure to direct sunlight as
much as possible. At low temperatures
and in the presence of humidity, formation
of ice could reduce flexibility and increase
cutting and abrasion-related risks on
textile and synthetic devices,
- Report to the manufacturer and the
competent authority of the member
state where the user and / or patient is
established any serious incident occurring
in relation to the MD.
All our devices are tested/ checked piece
by piece in compliance with the procedures
laid down by the Quality System certified
in accordance with the UNI EN ISO
9001 standard. Laboratory tests, testing,
information and standards do not always
reproduce the practical result. Thus, the
results obtained under the actual conditions
of use of the device in the natural environment
may differ, even considerably at times.
The best information lies in the continuous
practical use under the supervision of skilled/
expert/qualified people.
CHAPTER 2

6
original version in Italian dated 10/01/2022
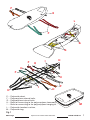
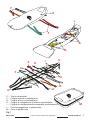
A – Aluminium alloy heads,
B – Aluminium alloy longitudinal beams,
C1 and C2 – Aluminium alloy handles,
D – Foam elastomer handle padding covered in polyester.
The scoop stretcher is provided, and can be kept away in the user’s bag, with the sheet
connected to the framework so as to facilitate preparation to use.
The parts, illustrated in paragraph 3.1, can still be separated from each other.
TECHNICAL
FEATURES
3.1 TERMINOLOGY AND MATERIALS OF THE PARTS
Fig. 1
CHAPTER 3
C2
B
B
B
B
B
DD
D
D
B
A
A
A
A
C1

870.04 LECCO 2.0www.kong.it
7
original version in Italian dated 10/01/2022
E – Polyamide sheet,
F – Polypropylene external belts,
G – Polypropylene internal belts,
H – Belts for connecting to the polypropylene framework,
I – Belts for connecting to the polypropylene hanging kit ,
L – Polyamide headrest cushion,
M – Polyamide bag.
Fig. 1
M
F
F
E
G
G
L
H
H
H
I
I
H
HH
F
F
F

8
original version in Italian dated 10/01/2022
3.2 TECHNICAL DATA
Length: 198 cm (without handles) 310 cm (with handles)
Width: 46.5 cm
Overall weight: 16.9 kg (excluding backpack) 19.2 kg (including backpack)
Capacity 300 kg*
3.3 OPTIONAL COMPONENTS AND SPARE PARTS
3.3.1 Optional components
871.13 ORION 2.0
Hanging kit with coloured ends (red, head side - blue, feet side) and connectors with twist
lock sleeve, suitable for lifting and lowering using rescue devices and helicopters.
The methods of connection and use of the optional components are defined in the relative
user instructions.
*For helicopter hoisting and lowering operations, given the 1:14 safety factor, the capacity
is reduced to 150 kg.
Fig. 2

870.04 LECCO 2.0www.kong.it
9
original version in Italian dated 10/01/2022
Fig. 3
871.04 EVOLUTION
Two-piece hanging kit with coloured ends (red, head side - blue, feet side) and connectors
with twist lock sleeve, suitable for lifting and lowering using rescue devices and helicopters.
871.01 GEMINI
Hanging kit with coloured ends (red, head side - blue, feet side) and connectors with twist
lock sleeve, suitable for transporting the scoop stretcher on ropeway conveyor and
overhead lines.
Fig. 4

10
original version in Italian dated 10/01/2022
871.02 VIRGO
High-load-capacity belt, to be connected to the rope, for fastening when lowering and
rescuing on areas with steep inclinations and in the space.
Fig. 5
871.03 AQUARIUS
Kit for fixing the scoop stretcher to the deck of the helicopters. Consisting of 4 length-wise
adjustable webbings with oval links and quick-release stainless steel connectors.
Fig. 6

870.04 LECCO 2.0www.kong.it
11
original version in Italian dated 10/01/2022
871.20 WILLY
Height-wise adjustable wheel with low-pressure tyre to be applied to the stretcher in a
barycentric position. It allows the transportation of the patient on flat grounds or on mule
tracks.
Fig. 8
871150000KK LECCO X-TENSION
Kit consisting of 4 polyamide straps necessary to connect the stretcher, with the X-TRIM
spinal board inserted, to the hanging kit.
Fig. 7

12
original version in Italian dated 10/01/2022
871.31 VISI
Lexan® face protection visor.
Fig. 10
871.22 TWIN WILLY
Height-wise adjustable twinned wheel with low-pressure tyres to be applied to the stretcher
in a barycentric position. It allows the transportation of the patient on flat grounds or on
mule tracks. Can be combined with the 871.20 WILLY single wheel.
Fig. 9

870.04 LECCO 2.0www.kong.it
13
original version in Italian dated 10/01/2022
871.51 GRIPS
Quick coupling grippers for moving the scoop stretcher in narrow spaces.
871.50 HANDLES
Quick-coupling handles for transporting the stretcher using the 871.20 WILLY and 871.22
TWIN WILLY wheels. Also suitable for shoulder-transportation carried by four rescue
operators.
Fig. 12
Fig. 11

14
original version in Italian dated 10/01/2022
871.41 WRAP
Backbone protection for transportation and sliding on snowy slopes.
Fig. 13

870.04 LECCO 2.0www.kong.it
15
original version in Italian dated 10/01/2022
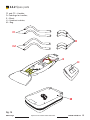
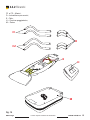
3.3.2 Spare parts
C1 and C2 – Handles,
D– Paddings for handles,
E – Sheet,
H – Headrest cushion,
M – Bag.
Fig. 14
D
H
M
E
C1
C2

16
original version in Italian dated 10/01/2022
SPECIFIC
INFORMATION
4.1 INTENDED USE
The “LECCO 2.0” stretcher is a medical device, particularly suitable for mountain rescue
operations, for rescuing and transporting a patient, even immobilised on “X-TRIM” spinal
boards or on “VACUUM” mattresses.
Decisions concerning moving and immobilising the patient, as well as the duration, method
to be used and combination with other devices, shall be taken and executed by expert and
trained personnel only.
The 871.13 ORION 2.0 and 871.04 EVOLUTION hanging kits, not part of standard supply,
make the stretcher utilisable with winch.
CHAPTER 4

870.04 LECCO 2.0www.kong.it
17
original version in Italian dated 10/01/2022
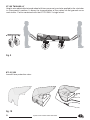
4.2 PREPARING THE STRETCHER
a) Take the pre-assembled stretcher from the bag and spread it with the sheet facing
towards the ground,
b) connect the central longitudinal beams (A1) to the longitudinal beams of the head element
(A2) and subsequently to the longitudinal beams of the other head element.
To connect the longitudinal beams:
- keep the pin (N) pressed,
- insert the central longitudinal beam into the longitudinal beam of the head element,
- check whether the pin (N) is automatically inserted into the hole of the longitudinal
beam of the head element.
The resistance of the stretcher is guaranteed by the correct assembly and
stretching of the sheet belts. The pins (N) are provided solely for facilitating
assembly.
Fig. 15
N
N
A2A2
A1 A1
A2A2

18
original version in Italian dated 10/01/2022
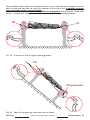
c) Slightly tighten the transversal belts,
d) slightly tighten the longitudinal belts being keen not to misalign the transversal belts,
Fig. 17
Fig. 16

870.04 LECCO 2.0www.kong.it
19
original version in Italian dated 10/01/2022
e) tighten the transversal belts definitely, insert the surplus part into the through element
and position it between the sheet and the stretched belt,
f) stretch the longitudinal belts definitely, insert the surplus part into the through element
and position it between the sheet and the stretched belt.
Fig. 19
Fig. 18

20
original version in Italian dated 10/01/2022
4.3 POSITIONING AND IMMOBILISING THE PATIENT
With the stretcher positioned on a flat and stable surface:
a) open the sheet zip fully and release the buckles of the internal belts,
b) lay the patient on the stretcher, couple the buckles and stretch the belts until the patient is
immobilised.
Avoid prolonged contact of the patient’s skin with the fabrics of the device
Fig. 20
Fig. 21
This procedure shall be applied even when patients are immobilised in “X-TRIM”
spinal boards (except for the groin belt) or with “VACUUM” mattresses.
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
La pagina si sta caricando...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
-
 45
45
-
 46
46
-
 47
47
-
 48
48
-
 49
49
-
 50
50
-
 51
51
-
 52
52
-
 53
53
-
 54
54
-
 55
55
-
 56
56
-
 57
57
-
 58
58
-
 59
59
-
 60
60
-
 61
61
-
 62
62
-
 63
63
-
 64
64
-
 65
65
-
 66
66
-
 67
67
-
 68
68
-
 69
69
-
 70
70
-
 71
71
-
 72
72
-
 73
73
-
 74
74
-
 75
75
-
 76
76
-
 77
77
-
 78
78
-
 79
79
-
 80
80
in altre lingue
- English: Kong Lecco 2.0 Tactical User manual
Documenti correlati
-
 Kong X-TRIM 4 Manuale utente
Kong X-TRIM 4 Manuale utente
-
 Kong 911 Hive Manuale utente
Kong 911 Hive Manuale utente
-
 Kong Vacuum 4 Manuale utente
Kong Vacuum 4 Manuale utente
-
 Kong 911 Armor Manuale utente
Kong 911 Armor Manuale utente
-
 Kong 911 CANYON Manuale utente
Kong 911 CANYON Manuale utente
-
 Kong Half Rolly Manuale utente
Kong Half Rolly Manuale utente
-
 Kong Kit Everest Carbon Tactical Manuale utente
Kong Kit Everest Carbon Tactical Manuale utente
-
 Kong X-TWO (AW169) Manuale utente
Kong X-TWO (AW169) Manuale utente
-
 Kong ORTLES Manuale utente
Kong ORTLES Manuale utente
-
 Kong RAIZER Manuale utente
Kong RAIZER Manuale utente



























































































